Stabilizing the integrin alpha M inserted domain in alternative conformations with a range of engineered disulfide bonds
- PMID: 12466503
- PMCID: PMC139213
- DOI: 10.1073/pnas.252633099
Stabilizing the integrin alpha M inserted domain in alternative conformations with a range of engineered disulfide bonds
Abstract
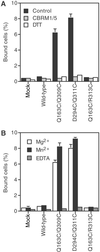
Conformational movement of the C-terminal alpha7 helix in the integrin inserted (I) domain, a major ligand-binding domain that adopts an alpha/beta Rossmann fold, has been proposed to allosterically regulate ligand-binding activity. Disulfide bonds were engineered here to reversibly lock the position of the alpha7 helix in one of two alternative conformations seen in crystal structures, termed open and closed. Our results show that pairs of residues with Cbeta atoms farther apart than optimal for disulfide bond stereochemistry can be successfully replaced by cysteine, suggesting that backbone movement accommodates disulfide formation. We also find more success with substituting partially exposed than buried residues. Disulfides stabilizing the open conformation resulted in constitutively active alphaMbeta2 heterodimers and isolated alphaM inserted domains, which were reverted to an inactive form by dithiothreitol reduction. By contrast, a disulfide stabilizing the closed conformation resulted in inactive alphaMbeta2 that was resistant to activation but became activatable after dithiothreitol treatment.
Figures



Similar articles
-
Reversibly locking a protein fold in an active conformation with a disulfide bond: integrin alphaL I domains with high affinity and antagonist activity in vivo.Proc Natl Acad Sci U S A. 2001 May 22;98(11):6009-14. doi: 10.1073/pnas.101130498. Epub 2001 May 15. Proc Natl Acad Sci U S A. 2001. PMID: 11353828 Free PMC article.
-
An isolated, surface-expressed I domain of the integrin alphaLbeta2 is sufficient for strong adhesive function when locked in the open conformation with a disulfide bond.Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2387-92. doi: 10.1073/pnas.041606398. Proc Natl Acad Sci U S A. 2001. PMID: 11226249 Free PMC article.
-
Engineered allosteric mutants of the integrin alphaMbeta2 I domain: structural and functional studies.Biochem J. 2003 May 15;372(Pt 1):121-7. doi: 10.1042/BJ20021273. Biochem J. 2003. PMID: 12611591 Free PMC article.
-
Conformational regulation of integrin structure and function.Annu Rev Biophys Biomol Struct. 2002;31:485-516. doi: 10.1146/annurev.biophys.31.101101.140922. Epub 2001 Oct 25. Annu Rev Biophys Biomol Struct. 2002. PMID: 11988479 Review.
-
Integrin activation and structural rearrangement.Immunol Rev. 2002 Aug;186:141-63. doi: 10.1034/j.1600-065x.2002.18613.x. Immunol Rev. 2002. PMID: 12234369 Review.
Cited by
-
Targeted molecular dynamics reveals overall common conformational changes upon hybrid domain swing-out in beta3 integrins.Proteins. 2009 Nov 1;77(2):477-89. doi: 10.1002/prot.22463. Proteins. 2009. PMID: 19455709 Free PMC article.
-
Anthrax toxin receptor 1/tumor endothelial marker 8: mutation of conserved inserted domain residues overrides cytosolic control of protective antigen binding.Biochemistry. 2010 Aug 31;49(34):7403-10. doi: 10.1021/bi100887w. Biochemistry. 2010. PMID: 20690680 Free PMC article.
-
Identification of novel agonists of the integrin CD11b/CD18.Bioorg Med Chem Lett. 2009 Dec 15;19(24):6902-6. doi: 10.1016/j.bmcl.2009.10.077. Epub 2009 Oct 22. Bioorg Med Chem Lett. 2009. PMID: 19879752 Free PMC article.
-
The crystal structure of C2a, the catalytic fragment of classical pathway C3 and C5 convertase of human complement.J Mol Biol. 2007 Mar 16;367(1):224-33. doi: 10.1016/j.jmb.2006.12.039. Epub 2006 Dec 19. J Mol Biol. 2007. PMID: 17234210 Free PMC article.
-
The Q163C/Q309C mutant of αMI-domain is an active variant suitable for NMR characterization.PLoS One. 2023 Jan 25;18(1):e0280778. doi: 10.1371/journal.pone.0280778. eCollection 2023. PLoS One. 2023. PMID: 36696377 Free PMC article.
References
-
- Humphries M. J. (2000) Biochem. Soc. Trans. 28, 311-339. - PubMed
-
- Shimaoka M., Takagi, J. & Springer, T. A. (2002) Annu. Rev. Biophys. Biomol. Struct. 31, 485-516. - PubMed
-
- Lee J.-O., Rieu, P., Arnaout, M. A. & Liddington, R. (1995) Cell 80, 631-638. - PubMed
-
- Lee J.-O., Bankston, L. A., Arnaout, M. A. & Liddington, R. C. (1995) Structure (London) 3, 1333-1340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

