Stringent doxycycline dependent control of CRE recombinase in vivo
- PMID: 12466566
- PMCID: PMC137989
- DOI: 10.1093/nar/gnf134
Stringent doxycycline dependent control of CRE recombinase in vivo
Abstract
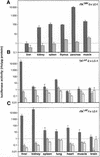
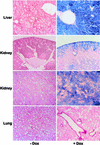
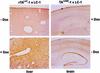
The strategy of modulating gene activities in vivo via CRE/loxP recombination would greatly profit from subjecting the recombination event to an independent and stringent temporal control. Here, we describe a transgenic mouse line, LC-1, where the expression of the cre and luciferase gene is tightly controlled by the Tet system. Using the R26R mouse line as indicator for CRE activity, and mouse lines expressing tetracycline controlled transactivators (tTA/rtTA) in various tissues, we show that; (i) in the non-induced state CRE recombinase is tightly controlled throughout the development and adulthood of an animal; (ii) upon induction, efficient recombination occurs in the adult animal in all tissues where tTA/rtTA is present, including hepatocytes, kidney cells, neurons and T lymphocytes; and (iii) no position effect appears to be caused by the LC-1 locus. Moreover, using the novel rTA(LAP)-1 mouse line, we show that in hepatocytes, complete deletion of the loxP-flanked insert in R26R animals is achieved less than 48 h after induction. Thus, the LC-1 mouse appears suitable for exploiting two rapidly increasing collections of mouse lines of which one provides tTA/rtTA in specific cell types/tissues, and the other a variety of loxP-flanked genes.
Figures



References
-
- Lewandoski M. (2001) Conditional control of gene expression in the mouse. Nature Rev. Genet., 2, 743–755. - PubMed
-
- Kühn R. and Schwenk,F. (2002) Conditional knockout mice. In van Deursen,J. and Hofker,M. (eds), The Transgenic Mouse: Methods and Protocols. Humana Press Inc., Totowa, NJ, USA, pp. 159–186.
-
- Thyagarajan B., Guimaraes,M.J., Groth,A.C. and Calos,M.P. (2000) Mammalian genomes contain active recombinase recognition sites. Gene, 244, 47–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

