Establishment and characterization of an immortalized Z310 choroidal epithelial cell line from murine choroid plexus
- PMID: 12470873
- PMCID: PMC3980880
- DOI: 10.1016/s0006-8993(02)03683-1
Establishment and characterization of an immortalized Z310 choroidal epithelial cell line from murine choroid plexus
Abstract
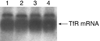
The choroid plexus plays a wide range of roles in brain development, maturation, aging process, endocrine regulation, and pathogenesis of certain neurodegenerative diseases. To facilitate in vitro study, we have used a gene transfection technique to immortalize murine choroidal epithelial cells. A viral plasmid (pSV3neo) was inserted into the host genome of primary choroidal epithelia by calcium phosphate precipitation. The transfected epithelial cells, i.e., Z310 cells, that survived from cytotoxic selection expressed SV40 large-T antigen throughout the life span, suggesting a successful gene transfection. The cells displayed the same polygonal epithelial morphology as the starting cells by light microscopy. Immunocytochemical studies demonstrate the presence of transthyretin (TTR), a thyroxine transport protein known to be exclusively produced by the choroidal epithelia in the CNS, in both transfected and starting cells. Western blot analyses further confirm the production and secretion of TTR by these cells. The mRNAs encoding transferrin receptor (TfR) were identified by Northern blot analyses. The cells grow at a steady rate, currently in the 110th passage with a population doubling time of 20-22 h in the established culture. When Z310 cells were cultured onto a Trans-well apparatus, the cells formed an epithelial monolayer similar to primary choroidal cells, possessing features such as an uneven fluid level between inner and outer chambers and an electrical resistance approximately 150-200 omega-cm(2). These results indicate that immortalized Z310 cells possess the characteristics of choroidal epithelia and may have the potential for application in blood-CSF barrier (BCB) research.
Copyright 2002 Elsevier Science B.V.
Figures






References
-
- Chodobski A, Szmydynger-Chodobska J. Choroid plexus: target for polypeptides and site of their synthesis. Microsc. Res. Tech. 2001;52:65–82. - PubMed
-
- Connor JR, Benkovic SA. Iron regulation in the brain: histochemical, biochemical, and molecular considerations. Ann. Neurol. 1992;32(Suppl):S51–S61. - PubMed
-
- Crook RB, Kasagami H, Prusiner SB. Culture and characterization of epithelial cells from bovine choroid plexus. J. Neurochem. 1981;37:845–854. - PubMed
-
- Davson H, Segal MB. Physiology of the CSF and Blood–Brain Barriers. New York: CRC Press; 1996. pp. 25–47.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

