G protein-coupled receptor rhodopsin: a prospectus
- PMID: 12471166
- PMCID: PMC1435697
- DOI: 10.1146/annurev.physiol.65.092101.142611
G protein-coupled receptor rhodopsin: a prospectus
Abstract
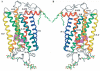
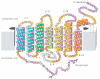
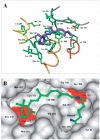
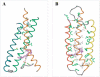
Rhodopsin is a retinal photoreceptor protein of bipartite structure consisting of the transmembrane protein opsin and a light-sensitive chromophore 11-cis-retinal, linked to opsin via a protonated Schiff base. Studies on rhodopsin have unveiled many structural and functional features that are common to a large and pharmacologically important group of proteins from the G protein-coupled receptor (GPCR) superfamily, of which rhodopsin is the best-studied member. In this work, we focus on structural features of rhodopsin as revealed by many biochemical and structural investigations. In particular, the high-resolution structure of bovine rhodopsin provides a template for understanding how GPCRs work. We describe the sensitivity and complexity of rhodopsin that lead to its important role in vision.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

