Lamin A/C speckles mediate spatial organization of splicing factor compartments and RNA polymerase II transcription
- PMID: 12473687
- PMCID: PMC2173379
- DOI: 10.1083/jcb.200204149
Lamin A/C speckles mediate spatial organization of splicing factor compartments and RNA polymerase II transcription
Abstract
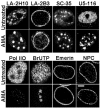
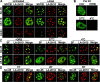
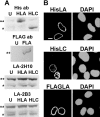
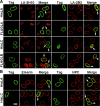
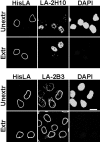
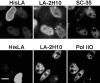
The A-type lamins have been observed to colocalize with RNA splicing factors in speckles within the nucleus, in addition to their typical distribution at the nuclear periphery. To understand the functions of lamin speckles, the effects of transcriptional inhibitors known to modify RNA splicing factor compartments (SFCs) were examined. Treatment of HeLa cells with alpha-amanitin or 5,6-dichlorobenzimidazole riboside (DRB) inhibited RNA polymerase II (pol II) transcription and led to the enlargement of lamin speckles as well as SFCs. Removal of the reversible inhibitor DRB resulted in the reactivation of transcription and a rapid, synchronous redistribution of lamins and splicing factors to normal-sized speckles, indicating a close association between lamin speckles and SFCs. Conversely, the expression of NH2-terminally modified lamin A or C in HeLa cells brought about a loss of lamin speckles, depletion of SFCs, and down-regulation of pol II transcription without affecting the peripheral lamina. Our results suggest a unique role for lamin speckles in the spatial organization of RNA splicing factors and pol II transcription in the nucleus.
Figures

References
-
- Beyer, A.L., N. Yvonne, and Y.N. Osheim. 1988. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 2:754–765. - PubMed
-
- Bonne, G., M.R. Di Barletta, S. Varnous, H.M. Becane, E.H. Hammonda, L. Merlini, F. Muntoni, C.R. Greenberg, F. Gary, J.A. Urtizberea, et al. 1999. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 21:285–288. - PubMed
-
- Bridger, J.M., I.R. Kill, M. O'Farrell, and C.J. Hutchison. 1993. Internal lamin structures within G1 nuclei of human dermal fibroblasts. J. Cell Sci. 104:297–306. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

