A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins
- PMID: 12475965
- PMCID: PMC138646
- DOI: 10.1091/mbc.e02-05-0311
A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins
Abstract
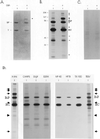
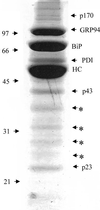
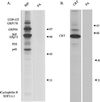
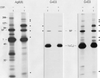
We demonstrate the existence of a large endoplasmic reticulum (ER)-localized multiprotein complex that is comprised of the molecular chaperones BiP; GRP94; CaBP1; protein disulfide isomerase (PDI); ERdj3, a recently identified ER Hsp40 cochaperone; cyclophilin B; ERp72; GRP170; UDP-glucosyltransferase; and SDF2-L1. This complex is associated with unassembled, incompletely folded immunoglobulin heavy chains. Except for ERdj3, and to a lesser extent PDI, this complex also forms in the absence of nascent protein synthesis and is found in a variety of cell types. Cross-linking studies reveal that the majority of these chaperones are included in the complex. Our data suggest that this subset of ER chaperones forms an ER network that can bind to unfolded protein substrates instead of existing as free pools that assembled onto substrate proteins. It is noticeable that most of the components of the calnexin/calreticulin system, which include some of the most abundant chaperones inside the ER, are either not detected in this complex or only very poorly represented. This study demonstrates an organization of ER chaperones and folding enzymes that has not been previously appreciated and suggests a spatial separation of the two chaperone systems that may account for the temporal interactions observed in other studies.
Figures




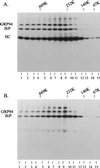
References
-
- Balow JP, Weissman JD, Kearse KP. Unique expression of major histocompatibility complex class I proteins in the absence of glucose trimming and calnexin association. J Biol Chem. 1995;270:29025–29029. - PubMed
-
- Bies C, Guth S, Janoschek K, Nastainczyk W, Volkmer J, Zimmermann R. A Scj1p homolog and folding catalysts present in dog pancreas microsomes. Biol Chem. 1999;380:1175–1182. - PubMed
-
- Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, Gething MJ. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993;75:717–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

