Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: phosphate substitution leads to multiple pathways of inhibition of platelet aggregation
- PMID: 12477353
- PMCID: PMC9233741
- DOI: 10.1021/jm020173u
Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: phosphate substitution leads to multiple pathways of inhibition of platelet aggregation
Abstract
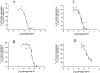
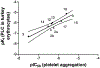
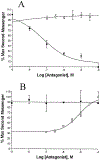
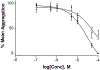
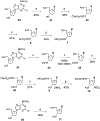
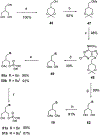
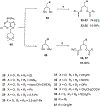
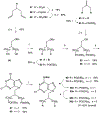
Activation by ADP of both P2Y(1) and P2Y(12) receptors in platelets contributes to platelet aggregation, and antagonists at these receptor subtypes have antithrombotic properties. In an earlier publication, we have characterized the SAR as P2Y(1) receptor antagonists of acyclic analogues of adenine nucleotides, containing two phosphate groups on a symmetrically branched aliphatic chain, attached at the 9-position of adenine. In this study, we have focused on antiaggregatory effects of P2Y antagonists related to a 2-chloro-N(6)-methyladenine-9-(2-methylpropyl) scaffold, containing uncharged substitutions of the phosphate groups. For the known nucleotide (cyclic and acyclic) bisphosphate antagonists of P2Y(1) receptors, there was a significant correlation between inhibition of aggregation induced by 3.3 microM ADP in rat platelets and inhibition of P2Y(1) receptor-induced phospholipase C (PLC) activity previously determined in turkey erythrocytes. Substitution of the phosphate groups with nonhydrolyzable phosphonate groups preserved platelet antiaggregatory activity. Substitution of one of the phosphate groups with O-acyl greatly reduced the inhibitory potency, which tended to increase upon replacement of both phosphate moieties of the acyclic derivatives with uncharged (e.g., ester) groups. In the series of nonsymmetrically substituted monophosphates, the optimal antagonist potency occurred with the phenylcarbamate group. Among symmetrical diester derivatives, the optimal antagonist potency occurred with the di(phenylacetyl) group. A dipivaloyl derivative, a representative uncharged diester, inhibited ADP-induced aggregation in both rat (K(I) 3.6 microM) and human platelets. It antagonized the ADP-induced inhibition of the cyclic AMP pathway in rat platelets (IC(50) 7 microM) but did not affect hP2Y(1) receptor-induced PLC activity measured in transfected astrocytoma cells. We propose that the uncharged derivatives are acting as antagonists of a parallel pro-aggregatory receptor present on platelets, that is, the P2Y(12) receptor. Thus, different substitution of the same nucleoside scaffold can target either of two P2Y receptors in platelets.
Figures

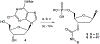

References
-
- Di Virgilio F; Chiozzi P; Ferrari D; Falzoni S; Sanz JM; Morelli A; Torboli M; Bolognesi G; Baricordi OR Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 2001, 97, 587–600. - PubMed
-
- Burnstock G; Williams M P2 Purinergic Receptors: Modulation of Cell Function and Therapeutic Potential. J. Pharmacol. Exp. Ther 2000, 295, 862–869. - PubMed
-
- Jin J; Daniel JL; Kunapuli SP Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J. Biol. Chem 1998, 273, 2030–2034. - PubMed
-
- Hechler B; Leon C; Vial C; Vigne P; Frelin C; Cazenave JP; Gachet C The P2Y1 receptor is necessary for adenosine 5_-diphosphate-induced platelet aggregation. Blood 1998, 92, 152–159. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

