Finasteride in the treatment of clinical benign prostatic hyperplasia: a systematic review of randomised trials
- PMID: 12477383
- PMCID: PMC140032
- DOI: 10.1186/1471-2490-2-14
Finasteride in the treatment of clinical benign prostatic hyperplasia: a systematic review of randomised trials
Abstract
Background: Benign prostatic hyperplasia affects older men. This systematic review determined efficacy and adverse effects of finasteride.
Review methods: PubMed, the Cochrane Library, reference lists of reports, and reviews were searched for randomised, double-blind trials of finasteride in benign prostatic hyperplasia. Outcomes included symptom score, urinary flow rate, prostate volume, discontinuation, and adverse effects. Relative risk and NNT or NNH were calculated for dichotomous data. Sensitivity analyses assessed influences of baseline symptom severity, initial prostate volume, a dominating trial, and previous interventions.
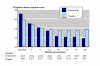
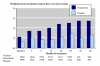
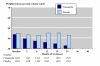
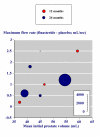
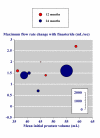
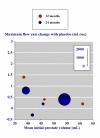
Results: Three trials had active controls and 19 had placebo. In placebo-controlled trials, 8820 patients received finasteride 5 mg and 5909 placebo over 3-48 months. Over 48 months finasteride produced greater improvements in total symptom score, maximum urinary flow rate, and prostate volume. Significantly more sexual dysfunction, impotence, ejaculation disorder and decreased libido occurred with finasteride at 12 months; the NNH for any sexual dysfunction at 12 months was 14. Significantly fewer men treated with finasteride experienced acute retention or had surgery at 24 or 48 months than with placebo; at 12 months the NNT was 49 (31 to 112) to avoid one acute urinary retention and 31 (21 to 61) to avoid one surgery. Sensitivity analyses showed benefit with finasteride 5 mg to be constant irrespective of the initial prostate volume.
Conclusions: Information from many patients in studies of high quality showed beneficial effects of finasteride in terms of symptoms, flow rate and prostate volume. More utility would result if patient centred outcomes were reported in dichotomous form.
Figures
References
-
- Nordling J, Hald T. BPH versus LUTS: reflections on lower urinary tract symptoms in search of a definition of clinical benign prostatic hyperplasia. European Urology Update Series. 1997;6:54–60.
-
- Simpson RJ. Benign prostatic hyperplasia. An overview of epidemiology and treatment. Primary Care in the New NHS. 2001. pp. 184–186.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

