The Glc7p-interacting protein Bud14p attenuates polarized growth, pheromone response, and filamentous growth in Saccharomyces cerevisiae
- PMID: 12477789
- PMCID: PMC138766
- DOI: 10.1128/EC.1.6.884-894.2002
The Glc7p-interacting protein Bud14p attenuates polarized growth, pheromone response, and filamentous growth in Saccharomyces cerevisiae
Abstract
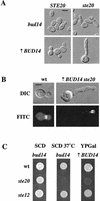
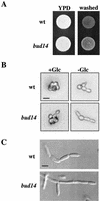
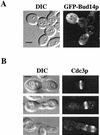
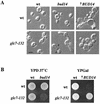
A genetic selection in Saccharomyces cerevisiae for mutants that stimulate the mating pathway uncovered a mutant that had a hyperactive pheromone response pathway and also had hyperpolarized growth. Cloning and segregation analysis demonstrated that BUD14 was the affected gene. Disruption of BUD14 in wild-type cells caused mild stimulation of pheromone response pathway reporters, an increase in sensitivity to mating factor, and a hyperelongated shmoo morphology. The bud14 mutant also had hyperfilamentous growth. Consistent with a role in the control of cell polarity, a Bud14p-green fluorescent protein fusion was localized to sites of polarized growth in the cell. Bud14p shared morphogenetic functions with the Ste20p and Bni1p proteins as well as with the type 1 phosphatase Glc7p. The genetic interactions between BUD14 and GLC7 suggested a role for Glc7p in filamentous growth, and Glc7p was found to have a positive function in filamentous growth in yeast.
Figures




References
-
- Andrews, P. D., and M. J. Stark. 2000. Type 1 protein phosphatase is required for maintenance of cell wall integrity, morphogenesis and cell cycle progression in Saccharomyces cerevisiae. J. Cell Sci. 113:507-520. - PubMed
-
- Bailis, J. M., and G. S. Roeder. 2000. Pachytene exit controlled by reversal of Mek1-dependent phosphorylation. Cell 101:211-221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

