Rvs161p and sphingolipids are required for actin repolarization following salt stress
- PMID: 12477802
- PMCID: PMC138763
- DOI: 10.1128/EC.1.6.1021-1031.2002
Rvs161p and sphingolipids are required for actin repolarization following salt stress
Abstract
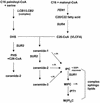
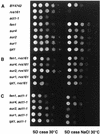
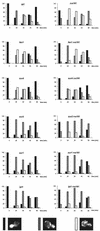
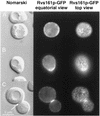
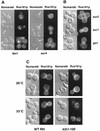
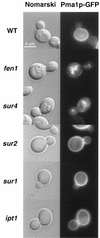
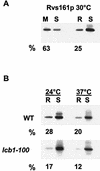
In Saccharomyces cerevisiae, the actin cytoskeleton is depolarized by NaCl stress. In this study, the response was maximal after 30 min, and then actin patches repolarized. Rvs161p was required for actin repolarization because the rvs161delta mutant did not repolarize actin patches after growth in a salt medium. Mutations suppressing the rvs161delta-related salt sensitivity all occurred in genes required for sphingolipid biosynthesis: FEN1, SUR4, SUR2, SUR1, and IPT1. These suppressors also suppressed act1-1-related salt sensitivity and the defect in actin repolarization of the rvs161delta mutant, providing a link between sphingolipids and actin polarization. Indeed, deletion of the suppressor genes suppressed the rvs161delta defect in actin repolarization in two ways: either actin was not depolarized at the wild-type level in a set of suppressor mutants, or actin was repolarized in the absence of Rvs161p in the other suppressor mutants. Rvs161p was localized as cortical patches that concentrated at polarization sites, i.e., bud emergence and septa, and was found to be associated with lipid rafts. An important link between sphingolipids and actin polarization is that Rvs161p was required for actin repolarization and was found to be located in lipid rafts.
Figures



References
-
- Bonneaud, N., O. Ozier-Kalogeropoulos, G. Y. Li, M. Labouesse, L. Minvielle-Sebastia, and F. Lacroute. 1991. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast 7:609-615. - PubMed
-
- Breton, A. M., and M. Aigle. 1998. Genetic and functional relationship between Rvsp, myosin and actin in Saccharomyces cerevisiae. Curr. Genet. 34:280-286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

