The major apoptotic pathway activated and suppressed by poliovirus
- PMID: 12477809
- PMCID: PMC140567
- DOI: 10.1128/jvi.77.1.45-56.2003
The major apoptotic pathway activated and suppressed by poliovirus
Abstract
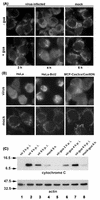
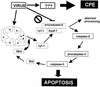
Cells respond to poliovirus infection by switching on the apoptotic program, implementation of which is usually suppressed by viral antiapoptotic functions. We show here that poliovirus infection of HeLa cells or derivatives of MCF-7 cells was accompanied by the efflux of cytochrome c from mitochondria. This efflux occurred during both abortive infection (e.g., interrupted by guanidine-HCl and ending with apoptosis) and productive infection (leading to cytopathic effect). The former type of infection, but not the latter, was accompanied by truncation of the proapoptotic protein Bid. The virus-triggered cytochrome c efflux was suppressed by overexpression of Bcl-2. Both abortive and productive infections also resulted in a decreased level of procaspase-9, as revealed by Western blotting. In the former case, this decrease was accompanied by the accumulation of a protein with the electrophoretic mobility of active caspase-9. In contrast, in the productively infected cells, the latter protein was absent but caspase-9-related polypeptides with altered mobility could be detected. Both caspase-9 and caspase-3 were shown to be essential for the development of such hallmarks of virus-induced apoptosis as chromatin condensation, DNA degradation, and nuclear fragmentation. These and some other results suggest the following scenario. Poliovirus infection activates the apoptotic pathway, involving mitochondrial damage, cytochrome c efflux, and consecutive activation of caspase-9 and caspase-3. The apoptotic signal appears to be amplified by a loop which includes secondary processing of Bid. The implementation of the apoptotic program in productively infected cells may be suppressed, however, by the viral antiapoptotic functions, which act at a step(s) downstream of the cytochrome c efflux. The suppression appears to be caused, at least in part, by aberrant processing and degradation of procaspase-9.
Figures





References
-
- Adams, J. M., and S. Cory. 2001. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 26:61-66. - PubMed
-
- Adrain, C., and S. J. Martin. 2001. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem. Sci. 26:390-397. - PubMed
-
- Agol, V. I., G. A. Belov, K. Bienz, D. Egger, M. S. Kolesnikova, N. T. Raikhlin, L. I. Romanova, E. A. Smirnova, and E. A. Tolskaya. 1998. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology 252:342-353. - PubMed
-
- Ammendolia, M. G., A. Tinari, A. Calcabrini, and F. Superti. 1999. Poliovirus infection induces apoptosis in CaCo-2 cells. J. Med. Virol. 59:122-129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

