Sequences downstream of the 5' splice donor site are required for both packaging and dimerization of human immunodeficiency virus type 1 RNA
- PMID: 12477813
- PMCID: PMC140568
- DOI: 10.1128/jvi.77.1.84-96.2003
Sequences downstream of the 5' splice donor site are required for both packaging and dimerization of human immunodeficiency virus type 1 RNA
Erratum in
- J Virol. 2003 Mar;77(6):3891.
Abstract
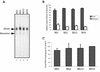
Two copies of human immunodeficiency virus type 1 RNA are incorporated into each virus particle and are further converted to a stable dimer as the virus particle matures. Several RNA segments that flank the 5' splice donor site at nucleotide (nt) 289 have been shown to act as packaging signals. Among these, RNA stem-loop 1 (SL1) (nt 243 to 277) can trigger RNA dimerization through a "kissing-loop" mechanism and thus is termed the dimerization initiation site. However, it is unknown whether other packaging signals are also needed for dimerization. To pursue this subject, we mutated stem-loop 3 (SL3) (nt 312 to 325), a GA-rich region (nt 325 to 336), and two G-rich repeats (nt 363 to 367 and nt 405 to 409) in proviral DNA and assessed the effects on RNA dimerization by performing native Northern blot analyses. Our results show that the structure but not the specific RNA sequence of SL3 is needed not only for efficient viral RNA packaging but also for dimerization. Mutations of the GA-rich sequence severely diminished viral RNA dimerization as well as packaging; the combination of mutations in both SL3 and the GA-rich region led to further decreases, implying independent roles for each of these two RNA motifs. Compensation studies further demonstrated that the RNA-packaging and dimerization activity of the GA-rich sequence may not depend on a putative interaction between this region and a CU repeat sequence at nt 227 to 233. In contrast, substitutions in the two G-rich sequences did not cause any diminution of viral RNA packaging or dimerization. We conclude that both the SL3 motif and GA-rich RNA sequences, located downstream of the 5' splice donor site, are required for efficient RNA packaging and dimerization.
Figures









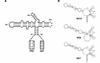
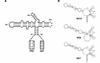


Similar articles
-
Compensatory point mutations in the human immunodeficiency virus type 1 Gag region that are distal from deletion mutations in the dimerization initiation site can restore viral replication.J Virol. 1998 Aug;72(8):6629-36. doi: 10.1128/JVI.72.8.6629-6636.1998. J Virol. 1998. PMID: 9658109 Free PMC article.
-
Mutational analysis of the 5' noncoding region of human immunodeficiency virus type 1 genome.Leukemia. 1997 Apr;11 Suppl 3:102-5. Leukemia. 1997. PMID: 9209312
-
Role of stem B, loop B, and nucleotides next to the primer binding site and the kissing-loop domain in human immunodeficiency virus type 1 replication and genomic-RNA dimerization.J Virol. 2001 Nov;75(21):10543-9. doi: 10.1128/JVI.75.21.10543-10549.2001. J Virol. 2001. PMID: 11581429 Free PMC article.
-
Dimerization of retroviral genomic RNAs: structural and functional implications.Biochimie. 1996;78(7):639-53. doi: 10.1016/s0300-9084(96)80010-1. Biochimie. 1996. PMID: 8955907 Review.
-
RNA Packaging in HIV.Trends Microbiol. 2019 Aug;27(8):715-723. doi: 10.1016/j.tim.2019.04.003. Epub 2019 May 10. Trends Microbiol. 2019. PMID: 31085095 Free PMC article. Review.
Cited by
-
Functional Equivalence of Retroviral MA Domains in Facilitating Psi RNA Binding Specificity by Gag.Viruses. 2016 Sep 19;8(9):256. doi: 10.3390/v8090256. Viruses. 2016. PMID: 27657107 Free PMC article.
-
A riboswitch regulates RNA dimerization and packaging in human immunodeficiency virus type 1 virions.J Virol. 2004 Oct;78(19):10814-9. doi: 10.1128/JVI.78.19.10814-10819.2004. J Virol. 2004. PMID: 15367648 Free PMC article.
-
HIV controls the selective packaging of genomic, spliced viral and cellular RNAs into virions through different mechanisms.Nucleic Acids Res. 2007;35(8):2695-704. doi: 10.1093/nar/gkm153. Epub 2007 Apr 10. Nucleic Acids Res. 2007. PMID: 17426127 Free PMC article.
-
Insulin-like growth factor II mRNA binding protein 1 associates with Gag protein of human immunodeficiency virus type 1, and its overexpression affects virus assembly.J Virol. 2008 Jun;82(12):5683-92. doi: 10.1128/JVI.00189-08. Epub 2008 Apr 2. J Virol. 2008. PMID: 18385235 Free PMC article.
-
An extended stem-loop 1 is necessary for human immunodeficiency virus type 2 replication and affects genomic RNA encapsidation.J Virol. 2007 Apr;81(7):3285-92. doi: 10.1128/JVI.02025-06. Epub 2007 Jan 17. J Virol. 2007. PMID: 17229705 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

