DNA immunization with hepatitis C virus (HCV) polycistronic genes or immunization by HCV DNA priming-recombinant canarypox virus boosting induces immune responses and protection from recombinant HCV-vaccinia virus infection in HLA-A2.1-transgenic mice
- PMID: 12477843
- PMCID: PMC140575
- DOI: 10.1128/jvi.77.1.382-390.2003
DNA immunization with hepatitis C virus (HCV) polycistronic genes or immunization by HCV DNA priming-recombinant canarypox virus boosting induces immune responses and protection from recombinant HCV-vaccinia virus infection in HLA-A2.1-transgenic mice
Abstract
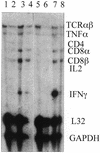
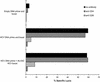
We studied immune responses to hepatitis C virus (HCV) genes delivered as DNA encoding the entire HCV protein coding genome in two polycistronic plasmids encoding HCV capsid-E1-E2-NS2-NS3 and HCV NS3-NS4-NS5 in HLA-A2.1-transgenic mice. Immune responses to HCV DNA prime and recombinant canarypox virus boost were also studied with the above constructs. At 8 weeks after a canarypox virus boost, the DNA prime/canarypox virus boosting regimen induced potent cellular immune responses to HCV structural and nonstructural proteins on target cells expressing the HLA-A2.1 allele. High frequencies of gamma interferon-secreting cells, as detected by enzyme-linked immunospot assay, were obtained in response to several endogenously expressed HCV proteins. We also observed cytotoxic-T-lymphocyte reactivity in response to endogenously expressed HCV proteins in fresh spleen cells without in vitro expansion. Upon challenge with a recombinant vaccinia virus expressing HCV proteins at 2 months postimmunization, the HCV DNA prime/canarypox virus-immunized mice showed a complete reduction in vaccinia virus titers compared to HCV DNA prime/boost- and mock-immunized controls. Immune responses were still detectable 4 months after canarypox virus boost in immunized mice. Interestingly, at 10 months postimmunization (8 months after canarypox virus boost), the protection in HCV DNA prime/boost-immunized mice against recombinant HCV-vaccinia virus challenge was higher than that observed in HCV DNA prime/canarypox virus boost-immunized mice.
Figures





References
-
- Allen, T. M., T. U Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. - PubMed
-
- Alter, M. J., H. S. Margolis, K. Krawczynski, et al. 1992. The natural history of community-acquired hepatitis in the United States: the sentinel counties chronic non-A, non-B hepatitis study team. N. Engl. J. Med. 327:1899-1905. - PubMed
-
- Arichi, T., T. Saito, M. E. Major, I. M. Belyakov, M. Shirai, V. H. Engelhard, S. M. Feinstone, and J. A. Berzofsky. 2000. Prophylactic DNA vaccine for hepatitis C virus (HCV) infection: HCV-specific cytotoxic T lymphocyte induction and protection from HCV-recombinant vaccinia infection in an HLA-A2.1 transgenic mouse model. Proc. Natl. Acad. Sci. USA 97:297-302. - PMC - PubMed
-
- Bukh, J., R. H. Miller, and R. H. Purcell. 1995. Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes. Semin. Liver Dis. 15:41-63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

