The ND10 component promyelocytic leukemia protein relocates to human papillomavirus type 1 E4 intranuclear inclusion bodies in cultured keratinocytes and in warts
- PMID: 12477870
- PMCID: PMC140640
- DOI: 10.1128/jvi.77.1.673-684.2003
The ND10 component promyelocytic leukemia protein relocates to human papillomavirus type 1 E4 intranuclear inclusion bodies in cultured keratinocytes and in warts
Abstract
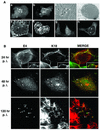
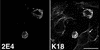
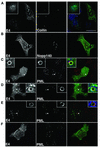
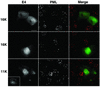
Human papillomavirus type 1 (HPV1) E4 protein is associated with cytoplasmic and nuclear inclusions in productively infected keratinocytes. Here we have used transient expression of HPV1 E4 (also known as E1E4) protein in keratinocytes to reproduce formation of E4 inclusions. Immunofluorescence analysis showed that progressive formation of inclusions correlated with diminished colocalization between E4 and keratin intermediate filaments (IFs). Our results support a model in which the HPV1 E4-keratin IF association is transient, occurring only at an early stage of inclusion formation. We also demonstrate that E4 induces relocation of the promyelocytic leukemia protein (PML) from multiple intranuclear speckles (ND10 bodies) to the periphery of nuclear E4 inclusions and that this activity is specific to full-length E4 protein. Analysis of HPV1-induced warts demonstrated that nuclear PML-E4 inclusions were present in productively infected keratinocytes, indicating that reorganization of PML occurs during the virus's replication cycle. It has been suggested that ND10 bodies are the sites for papillomavirus genome replication and virion assembly. Our finding that E4 induces reorganization of ND10 bodies in vitro and in vivo is further strong evidence that these domains play an important role in the papillomavirus life cycle. This study indicates that HPV1 is analogous to other DNA viruses that disrupt or reorganize ND10 domains, possibly to increase efficiency of virus infection. We hypothesize that HPV1 E4-induced reorganization of PML is necessary for efficient replication of the virus during the virus-producing phase.
Figures




Similar articles
-
Recruitment of human cytomegalovirus immediate-early 2 protein onto parental viral genomes in association with ND10 in live-infected cells.J Virol. 2007 Sep;81(18):10123-36. doi: 10.1128/JVI.01009-07. Epub 2007 Jul 11. J Virol. 2007. PMID: 17626080 Free PMC article.
-
Promyelocytic leukemia nuclear bodies provide a scaffold for human polyomavirus JC replication and are disrupted after development of viral inclusions in progressive multifocal leukoencephalopathy.J Neuropathol Exp Neurol. 2008 Apr;67(4):299-308. doi: 10.1097/NEN.0b013e31816a1dd3. J Neuropathol Exp Neurol. 2008. PMID: 18379438
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
Review: properties and assembly mechanisms of ND10, PML bodies, or PODs.J Struct Biol. 2000 Apr;129(2-3):278-87. doi: 10.1006/jsbi.2000.4239. J Struct Biol. 2000. PMID: 10806078 Review.
-
The use of fluorescence microscopy to study the association between herpesviruses and intrinsic resistance factors.Viruses. 2011 Dec;3(12):2412-24. doi: 10.3390/v3122412. Epub 2011 Dec 7. Viruses. 2011. PMID: 22355446 Free PMC article. Review.
Cited by
-
Cooperation between different forms of the human papillomavirus type 1 E4 protein to block cell cycle progression and cellular DNA synthesis.J Virol. 2004 Dec;78(24):13920-33. doi: 10.1128/JVI.78.24.13920-13933.2004. J Virol. 2004. PMID: 15564500 Free PMC article.
-
Human papillomavirus type 16 E1circumflexE4 contributes to multiple facets of the papillomavirus life cycle.J Virol. 2005 Oct;79(20):13150-65. doi: 10.1128/JVI.79.20.13150-13165.2005. J Virol. 2005. PMID: 16189016 Free PMC article.
-
CCCTC-binding factor recruitment to the early region of the human papillomavirus 18 genome regulates viral oncogene expression.J Virol. 2015 May;89(9):4770-85. doi: 10.1128/JVI.00097-15. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694598 Free PMC article.
-
STAT3 activation by E6 is essential for the differentiation-dependent HPV18 life cycle.PLoS Pathog. 2018 Apr 9;14(4):e1006975. doi: 10.1371/journal.ppat.1006975. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29630659 Free PMC article.
-
Human papillomavirus 18 E1^E4 protein interacts with cyclin A/CDK 2 through an RXL motif.Mol Cell Biochem. 2013 Jan;373(1-2):29-40. doi: 10.1007/s11010-012-1472-y. Epub 2012 Oct 13. Mol Cell Biochem. 2013. PMID: 23065011
References
-
- Ashmole, I., P. H. Gallimore, and S. Roberts. 1998. Identification of conserved hydrophobic C-terminal residues of the human papillomavirus type 1 E1^E4 protein necessary for E4 oligomerisation in vivo. Virology 240:221-231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

