The effect of rapid HIV-1 testing on uptake of perinatal HIV-1 interventions: a randomized clinical trial
- PMID: 12478076
- PMCID: PMC3380077
- DOI: 10.1097/00002030-200301030-00015
The effect of rapid HIV-1 testing on uptake of perinatal HIV-1 interventions: a randomized clinical trial
Abstract
Objective: We examined whether HIV-1 testing using a rapid assay increases the proportion of pregnant women obtaining HIV-1 results and the uptake of perinatal HIV-1 interventions.
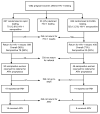
Methods: Pregnant women attending public health clinics in Nairobi were offered voluntary counselling and testing for HIV-1. Consenting women were randomly assigned to receive either rapid or conventional HIV-1 testing. Women randomly assigned to rapid testing were allowed to receive same-day results or to return later. The results for women randomly assigned to conventional enzyme-linked immunosorbent assay (ELISA) testing were available after 7 days. HIV-1-infected women were referred for antiretroviral prophylaxis to prevent mother-to-child transmission of HIV-1.
Results: Among 1282 women offered voluntary HIV-1 testing and counselling, 1249 accepted testing, of whom 627 were randomly assigned to rapid testing and 622 to conventional testing. The median duration between testing and obtaining results was 0 days for women who received rapid testing compared with 11 days for women who received conventional testing. The percentage receiving HIV-1 results was significantly higher among women who received rapid testing compared with conventional testing. Of 161 HIV-1-seropositive women, only 24 received antiretroviral prophylaxis. The uptake of perinatal HIV-1 interventions did not differ between HIV-1-seropositive women randomly assigned to rapid testing or conventional ELISA testing.
Conclusion: Rapid HIV-1 testing significantly increased the proportion of women receiving HIV-1 results, which is important for sexual and perinatal HIV-1 prevention. The challenge remains to improve the uptake of perinatal HIV-1 interventions among HIV-1-seropositive women.
Figures
References
-
- Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d’Ivoire: a randomized trial. Lancet. 1999;353:781–785. - PubMed
-
- Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NK, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353:773–780. - PubMed
-
- The PETRA Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda: a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–1186. - PubMed
-
- Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. - PubMed
-
- Dabis F, Newell ML, Fransen L, Saba J, Lepage P, Leroy V, et al. Prevention of mother-to-child transmission of HIV in developing countries: recommendations for practice. The Ghent International Working Group on Mother-To-Child Transmission of HIV. Health Policy Plan. 2000;15:34–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical