Potent suppression of viral infectivity by the peptides that inhibit multimerization of human immunodeficiency virus type 1 (HIV-1) Vif proteins
- PMID: 12480936
- PMCID: PMC1350967
- DOI: 10.1074/jbc.M210164200
Potent suppression of viral infectivity by the peptides that inhibit multimerization of human immunodeficiency virus type 1 (HIV-1) Vif proteins
Abstract
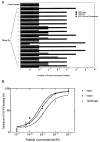
Virion infectivity factor (Vif) is essential for the replication of human immunodeficiency virus type 1 (HIV-1) in vivo, but its function remains uncertain. Recently, we have shown that Vif proteins are able to form multimers, including dimers, trimers, or tetramers. Because the multimerization of Vif proteins is required for Vif function in the viral life cycle, we propose that it could be a novel target for anti-HIV-1 therapeutics. Through a phage peptide display method, we have identified a set of 12-mer peptides containing a PXP motif that binds to HIV-1 Vif protein. These proline-enriched peptides potently inhibited the Vif-Vif interaction in vitro. We have also screened a set of synthesized Vif peptides (15-mer), which covers all the amino acids of the HIV-1 Vif protein sequence, for their ability to inhibit the Vif-Vif interaction in vitro. We demonstrated that Vif-derived proline-enriched peptides that contain the (161)PPLP(164) domain are able to inhibit the Vif-Vif interaction. Conversely, the deletion of the (161)PPLP(164) domain of Vif protein will significantly impair the capability of Vif proteins to interact with each other, indicating that the (161)PPLP(164) domain plays a key role in Vif multimerization. All these results demonstrate that the proline-enriched peptides block the multimerization of Vif through interfering with the polyproline interfaces of Vif formed by (161)PPLP(164) domain. Moreover, these peptides which inhibit the Vif-Vif interaction in vitro potently inhibit HIV-1 replication in the "nonpermissive" T-cells. We propose that this study starts a novel strategy to develop structural diverse inhibitors of Vif such as peptidomimetics or small organic molecules.
Figures

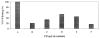


Similar articles
-
The multimerization of human immunodeficiency virus type I Vif protein: a requirement for Vif function in the viral life cycle.J Biol Chem. 2001 Feb 16;276(7):4889-93. doi: 10.1074/jbc.M004895200. Epub 2000 Nov 8. J Biol Chem. 2001. PMID: 11071884 Free PMC article.
-
The unique structure of the highly conserved PPLP region in HIV-1 Vif is critical for the formation of APOBEC3 recognition interfaces.mBio. 2025 Mar 12;16(3):e0333224. doi: 10.1128/mbio.03332-24. Epub 2025 Jan 21. mBio. 2025. PMID: 39835817 Free PMC article.
-
Human immunodeficiency virus type 1 Vif-derived peptides inhibit the viral protease and arrest virus production.FEBS Lett. 1998 Dec 28;441(3):419-26. doi: 10.1016/s0014-5793(98)01602-0. FEBS Lett. 1998. PMID: 9891983
-
Viral infectivity factor: a novel therapeutic strategy to block HIV-1 replication.Mini Rev Med Chem. 2013 Jun;13(7):1047-55. doi: 10.2174/1389557511313070008. Mini Rev Med Chem. 2013. PMID: 23621690 Review.
-
[Progress in the study of HIV-1 Vif and related inhibitors].Yao Xue Xue Bao. 2010 Jun;45(6):684-93. Yao Xue Xue Bao. 2010. PMID: 20939174 Review. Chinese.
Cited by
-
A New Class of Antiretroviral Enabling Innate Immunity by Protecting APOBEC3 from HIV Vif-Dependent Degradation.Trends Mol Med. 2018 May;24(5):507-520. doi: 10.1016/j.molmed.2018.03.004. Epub 2018 Mar 30. Trends Mol Med. 2018. PMID: 29609878 Free PMC article. Review.
-
Tumultuous relationship between the human immunodeficiency virus type 1 viral infectivity factor (Vif) and the human APOBEC-3G and APOBEC-3F restriction factors.Microbiol Mol Biol Rev. 2009 Jun;73(2):211-32. doi: 10.1128/MMBR.00040-08. Microbiol Mol Biol Rev. 2009. PMID: 19487726 Free PMC article. Review.
-
Multiple ways of targeting APOBEC3-virion infectivity factor interactions for anti-HIV-1 drug development.Trends Pharmacol Sci. 2009 Dec;30(12):638-46. doi: 10.1016/j.tips.2009.09.006. Trends Pharmacol Sci. 2009. PMID: 19837465 Free PMC article. Review.
-
The dimerization domain of HIV-1 viral infectivity factor Vif is required to block virion incorporation of APOBEC3G.Retrovirology. 2007 Nov 24;4:81. doi: 10.1186/1742-4690-4-81. Retrovirology. 2007. PMID: 18036235 Free PMC article.
-
Small-molecule inhibition of human immunodeficiency virus type 1 replication by targeting the interaction between Vif and ElonginC.J Virol. 2012 May;86(10):5497-507. doi: 10.1128/JVI.06957-11. Epub 2012 Feb 29. J Virol. 2012. PMID: 22379088 Free PMC article.
References
-
- Wieland U, Hartmann J, Suhr H, Salzberger B, Eggers HJ, Kuhn JE. Virology. 1994;203:43–51. - PubMed
-
- Strebel K, Daugherty D, Clouse K, Cohen D, Folks T, Martin MA. Nature. 1987;328:728–730. - PubMed
-
- Gabuzda DH, Li H, Lawrence K, Vasir BS, Crawford K, Langhoff E. J Acquired Immune Defic Syndr. 1994;7:908–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

