Molecular conformation of a peptide fragment of transthyretin in an amyloid fibril
- PMID: 12481032
- PMCID: PMC139215
- DOI: 10.1073/pnas.252625999
Molecular conformation of a peptide fragment of transthyretin in an amyloid fibril
Abstract
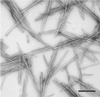
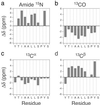
The molecular conformation of peptide fragment 105-115 of transthyretin, TTR(105-115), previously shown to form amyloid fibrils in vitro, has been determined by magic-angle spinning solid-state NMR spectroscopy. 13C and 15N linewidth measurements indicate that TTR(105-115) forms a highly ordered structure with each amino acid in a unique environment. 2D 13C-13C and 15N-13C-13C chemical shift correlation experiments, performed on three fibril samples uniformly 13C,15N-labeled in consecutive stretches of 4 aa, allowed the complete sequence-specific backbone and side-chain 13C and 15N resonance assignments to be obtained for residues 105-114. Analysis of the 15N, 13CO, 13Calpha, and 13Cbeta chemical shifts allowed quantitative predictions to be made for the backbone torsion angles phi and psi. Furthermore, four backbone 13C-15N distances were determined in two selectively 13C,15N-labeled fibril samples by using rotational-echo double-resonance NMR. The results show that TTR(105-115) adopts an extended beta-strand conformation that is similar to that found in the native protein except for substantial differences in the vicinity of the proline residue.
Figures

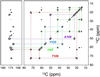

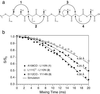
 ), L111 Cα-L110 N (○), and S112 CO-Y114 N (•). The measured distances are indicated in b and summarized in Table 3 (the S115 Cα-Y114 N measurement was omitted for clarity). Spectra were acquired with 320–384 transients and the spinning frequency of 10.0 kHz ± 5 Hz.
), L111 Cα-L110 N (○), and S112 CO-Y114 N (•). The measured distances are indicated in b and summarized in Table 3 (the S115 Cα-Y114 N measurement was omitted for clarity). Spectra were acquired with 320–384 transients and the spinning frequency of 10.0 kHz ± 5 Hz.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

