Small glutamine-rich protein/viral protein U-binding protein is a novel cochaperone that affects heat shock protein 70 activity
- PMID: 12482202
- PMCID: PMC514826
- DOI: 10.1379/1466-1268(2002)007<0258:sgrpvp>2.0.co;2
Small glutamine-rich protein/viral protein U-binding protein is a novel cochaperone that affects heat shock protein 70 activity
Abstract
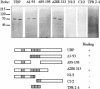
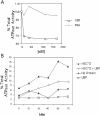
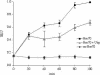
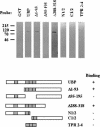
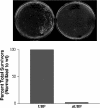
Molecular chaperone complexes containing heat shock protein (Hsp) 70 and Hsp90 are regulated by cochaperones, including a subclass of regulators, such as Hsp70 interacting protein (Hip), C-terminus of Hsp70 interacting protein (CHIP), and Hsp70-Hsp90 organizing factor (Hop), that contain tetratricopeptide repeats (TPRs), where Hsp70 refers to Hsp70 and its nearly identical constitutive counterpart, Hsc70, together. These proteins interact with the Hsp70 to regulate adenosine triphosphatase (ATPase) and folding activities or to generate the chaperone complex. Here we provide evidence that small glutamine-rich protein/viral protein U-binding protein (SGT/UBP) is a cochaperone that negatively regulates Hsp70. By "Far-Western" and pull-down assays, SGT/UBP was shown to interact directly with Hsp70 and weakly with Hsp90. The interaction of SGT/UBP with both these protein chaperones was mapped to 3 TPRs in SGT/UBP (amino acids 95-195) that are flanked by charged residues. Moreover, SGT/UBP caused an approximately 30% reduction in both the intrinsic ATPase activity of Hsc70 and the ability of Hsc70 to refold denatured luciferase in vitro. This negative effect of SGT/UBP on Hsc70 is similar in magnitude to that observed for the cochaperone CHIP. A role for SGT/UBP in protein folding is also supported by evidence that a yeast strain containing a deletion in the yeast homolog to SGT/UBP (delta SGT/UBP) displays a 50-fold reduction in recovery from heat shock compared with the wild type parent. Together, these results are consistent with a regulatory role for SGT/UBP in the chaperone complex.
Figures
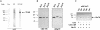

References
-
- Callahan MA, Handley MA, Lee YH, Talbot KJ, Harper JW, Panganiban AT. Functional interaction of human immunodeficiency virus type 1 Vpu and Gag with a novel member of the tetratricopeptide repeat protein family [published erratum appears in J Virol 1998 72: 8461] J Virol. 1998;72:5189–5197. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
