Heat shock protein-27 protects human bronchial epithelial cells against oxidative stress-mediated apoptosis: possible implication in asthma
- PMID: 12482203
- PMCID: PMC514827
- DOI: 10.1379/1466-1268(2002)007<0269:hspphb>2.0.co;2
Heat shock protein-27 protects human bronchial epithelial cells against oxidative stress-mediated apoptosis: possible implication in asthma
Abstract
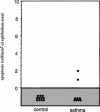
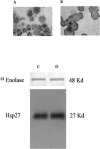
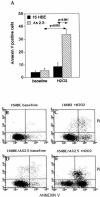
Inflammation of the human bronchial epithelium, as observed in asthmatics, is characterized by the selective death of the columnar epithelial cells, which desquamate from the basal cells. Tissue repair initiates from basal cells that resist inflammation. Here, we have evaluated the extent of apoptosis as well as the Hsp27 level of expression in epithelial cells from bronchial biopsy samples taken from normal and asthmatic subjects. Hsp27 is a chaperone whose expression protects against oxidative stress. We report that in asthmatic subjects the basal epithelium cells express a high level of Hsp27 but no apoptotic morphology. In contrast, apoptotic columnar cells are devoid of Hsp27 expression. Moreover, we observed a decreased resistance to hydrogen peroxide-induced apoptosis in human bronchial epithelial 16-HBE cells when they were genetically modified to express reduced levels of Hsp27.
Figures

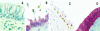


References
-
- Arata S, Hamaguchi S, Nose K. Effects of the overexpression of the small heat shock protein, Hsp27, on the sensitivity of human fibroblast cells exposed to oxidative stress. J Cell Physiol. 1995;163:458–465. - PubMed
-
- Aron Y, Busson M, Polla BS, Dusser D, Lockhart A, Swierczewski E, Favatier F. Analysis of hsp70 gene polymorphism in allergic asthma. Allergy. 1999;54:165–170. - PubMed
-
- Arrigo A-P. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. - PubMed
-
- Arrigo A-P. sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol Biol. 2000;48:280–288. - PubMed
-
- Arrigo A-P. Small stress proteins: novel regulators of intracellular redox state. IUBMB Life. 2002;52:303–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
