The relation between charge movement and transport-associated currents in the rat GABA cotransporter rGAT1
- PMID: 12482883
- PMCID: PMC2290732
- DOI: 10.1113/jphysiol.2002.026823
The relation between charge movement and transport-associated currents in the rat GABA cotransporter rGAT1
Abstract
Most cotransporters characteristically display two main kinds of electrical activity: in the absence of organic substrate, transient presteady-state currents (I(pre)) are generated by charge relocation during voltage steps; in the presence of substrate, sustained, transport-associated currents (I(tr)) are recorded. Quantitative comparison of these two currents, in Xenopus oocytes expressing the neural GABA cotransporter rGAT1, revealed several unforeseen consistencies between I(pre) and I(tr), in terms of magnitude and kinetic parameters. The decay rate constant (r) of I(pre) and the quantity of charge displaced to an inner position in the transporter (Q(in)(0)) depended on voltage and ionic conditions. Saturating GABA concentrations, applied under the same conditions, suppressed I(pre) (i.e. Q(in)( infinity ) = 0) and produced a transport-associated current with amplitude I(tr) = Q(in)(0)r. At non-saturating levels of GABA, changes of I(tr) were compensated by corresponding variations in Q(in), such that I(pre) and I(tr) complemented each other, according to the relation: I(tr) = (Q(in)(0) - Q(in)) r. Complementarity of magnitude, superimposable kinetic properties and equal dependence on voltage and [Na(+)](o) point to the uniqueness of the charge carrier for both processes, suggesting that transport and charge migration arise from the same molecular mechanism. The observed experimental relations were correctly predicted by a simple three-state kinetic model, in which GABA binding takes place after charge binding and inward migration have occurred. The model also predicts the observed voltage dependence of the apparent affinity of the transporter for GABA, and suggests a voltage-independent GABA binding rate with a value around 0.64 microM(-1) s(-1).
Figures

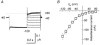
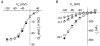
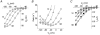
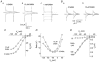



References
-
- Binda F, Bossi E, Giovannardi S, Forlani G, Peres A. Temperature effects on the presteady-state and transport-associated currents of GABA cotransporter rGAT1. FEBS Letters. 2002;512:303–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

