Regulation of Cl--HCO3- exchangers by cAMP-dependent protein kinase in adult rat hippocampal CA1 neurons
- PMID: 12482890
- PMCID: PMC2290728
- DOI: 10.1113/jphysiol.2002.027235
Regulation of Cl--HCO3- exchangers by cAMP-dependent protein kinase in adult rat hippocampal CA1 neurons
Abstract
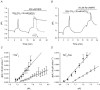
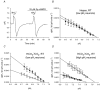
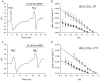
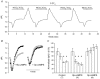
The contributions of HCO(3)(-)-dependent, DIDS-sensitive mechanisms to the maintenance of steady-state pH(i), and the regulation of their activities by cAMP-dependent protein kinase (PKA), were investigated in CA1 neurons with the H(+)-sensitive fluorophore, BCECF. The addition of HCO(3)(-)/CO(2) to neurons with "low" (pH(i) < or = 7.20) and "high" (pH(i) > 7.20) initial pH(i) values under Hepes-buffered conditions, increased and decreased steady-state pH(i), respectively. Conversely, under HCO(3)(-)/CO(2)-buffered conditions, DIDS caused pH(i) to decrease and increase in neurons with low and high initial pH(i) values, respectively. In the presence, but not the absence, of HCO(3)(-), the PKA inhibitor Rp-adenosine-3',5'-cyclic monophosphorothioate (Rp-cAMPS; 50 microM) evoked DIDS-sensitive increases and decreases in pH(i) in neurons with low and high initial pH(i) values, respectively. In contrast, in neurons with low initial pH(i) values, activation of PKA with the Sp isomer of cAMPS (Sp-cAMPS; 25 microM) elicited increases in pH(i) that were smaller in the presence than in the absence of HCO(3)(-), whereas in neurons with high initial pH(i) values, Sp-cAMPS-evoked rises in pH(i) were larger in the presence than in the absence of HCO(3)(-); the differences between the effects of Sp-cAMPS on pH(i) under the different buffering conditions were attenuated by DIDS. Consistent with the possibility that changes in the activities of HCO(3)(-)-dependent, DIDS-sensitive mechanisms contribute to the steady-state pH(i) changes evoked by the PKA modulators, in neurons with initial pH(i) values < or = 7.20, Rp-cAMPS concurrently inhibited Na(+)-independent Cl(-)-HCO(3)(-) exchange and stimulated Na(+)-dependent Cl(-)-HCO(3)(-) exchange; in contrast, Sp-cAMPS concurrently stimulated Na(+)-independent Cl(-)-HCO(3)(-) exchange and inhibited Na(+)-dependent Cl(-)-HCO(3)(-) exchange. Data from a limited number of neurons with initial pH(i) values > 7.20 suggested that the directions of the reciprocal changes in anion exchange activities (inhibition or stimulation) evoked by Rp- and Sp-cAMPS may be opposite in cells with low vs. high resting pH(i) values. Taken together, the results indicate that the effects of modulating PKA activity on steady-state pH(i) in rat CA1 neurons under HCO(3)(-)/CO(2)-buffered conditions reflect not only changes in Na(+)-H(+) exchange activity but also changes in Na(+)-dependent and Na(+)-independent Cl(-)-HCO(3)(-) exchange activity that, in turn, may be dependent upon the initial pH(i).
Figures


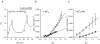
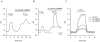
Similar articles
-
DIDS-sensitive pHi regulation in single rat cardiac myocytes in nominally HCO3-free conditions.Circ Res. 1994 Jul;75(1):123-32. doi: 10.1161/01.res.75.1.123. Circ Res. 1994. PMID: 8013070
-
Transporters involved in regulation of intracellular pH in primary cultured rat brain endothelial cells.J Physiol. 2006 Nov 1;576(Pt 3):769-85. doi: 10.1113/jphysiol.2006.117374. Epub 2006 Aug 17. J Physiol. 2006. PMID: 16916905 Free PMC article.
-
Basolateral membrane Cl(-)-, Na(+)-, and K(+)-coupled base transport mechanisms in rat MTALH.Am J Physiol Renal Physiol. 2002 Apr;282(4):F655-68. doi: 10.1152/ajprenal.00220.2000. Am J Physiol Renal Physiol. 2002. PMID: 11880327
-
Intracellular pH response to anoxia in acutely dissociated adult rat hippocampal CA1 neurons.J Neurophysiol. 2002 May;87(5):2209-24. doi: 10.1152/jn.2002.87.5.2209. J Neurophysiol. 2002. PMID: 11976362
-
Plasma membrane Cl⁻/HCO₃⁻ exchangers: structure, mechanism and physiology.Channels (Austin). 2008 Sep-Oct;2(5):337-45. doi: 10.4161/chan.2.5.6899. Channels (Austin). 2008. PMID: 19066446 Review.
Cited by
-
The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters.Physiol Rev. 2013 Apr;93(2):803-959. doi: 10.1152/physrev.00023.2012. Physiol Rev. 2013. PMID: 23589833 Free PMC article. Review.
-
Ouabain-induced stimulation of sodium-hydrogen exchange in rat optic nerve astrocytes.Am J Physiol Cell Physiol. 2008 Jul;295(1):C100-10. doi: 10.1152/ajpcell.90636.2007. Epub 2008 Apr 30. Am J Physiol Cell Physiol. 2008. PMID: 18448627 Free PMC article.
-
pH modulation of currents that contribute to the medium and slow afterhyperpolarizations in rat CA1 pyramidal neurones.J Physiol. 2004 Jan 15;554(Pt 2):449-66. doi: 10.1113/jphysiol.2003.051607. Epub 2003 Nov 7. J Physiol. 2004. PMID: 14608014 Free PMC article.
-
Regulation of human airway ciliary beat frequency by intracellular pH.J Physiol. 2004 Oct 15;560(Pt 2):519-32. doi: 10.1113/jphysiol.2004.068171. Epub 2004 Aug 12. J Physiol. 2004. PMID: 15308676 Free PMC article.
-
Effects of extracellular metabolic acidosis and out-of-equilibrium CO2/HCO3 - solutions on intracellular pH in cultured rat hippocampal neurons.Front Physiol. 2024 Oct 9;15:1434359. doi: 10.3389/fphys.2024.1434359. eCollection 2024. Front Physiol. 2024. PMID: 39444753 Free PMC article.
References
-
- Alvarez BV, Fujinaga J, Casey JR. Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circulation Research. 2001;89:1246–1253. - PubMed
-
- Autere A-M, Lamsa K, Kaila K, Taira T. Synaptic activation of GABAA receptors induces neuronal uptake of Ca2+ in adult rat hippocampal slices. Journal of Neurophysiology. 1999;81:811–816. - PubMed
-
- Boron WF, McCormick WC, Roos A. pH regulation in barNaCle muscle fibers: dependence on intracellular and extracellular pH. American Journal of Physiology. 1979;237:C185–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

