Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat
- PMID: 12482895
- PMCID: PMC2290729
- DOI: 10.1113/jphysiol.2002.024281
Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat
Abstract
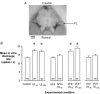
We investigated the role of the cerebellar flocculus in mediating the adaptive changes that occur in the intrinsic properties of brainstem medial vestibular nucleus (MVN) neurons during vestibular compensation. Ipsi-lesional, but not contra-lesional, flocculectomy prevented the compensatory increase in intrinsic excitability (CIE) that normally occurs in the de-afferented MVN neurons within 4 h after unilateral labyrinthectomy (UL). Flocculectomy did not, however, prevent the down-regulation of efficacy of GABA receptors that also occurs in these neurons after UL, indicating that these responses of the MVN neurons to deafferentation are discrete, parallel processes. CIE was also abolished by intra-floccular microinjection of the metabotropic glutamate receptor (mGluR) antagonist AIDA, and the protein kinase C inhibitor bisindolymaleimide I (BIS-I). The serene-threonine kinase inhibitor H-7 had no effect when microinjected at the time of de-afferentation, but abolished CIE if microinjected 2 h later. These cellular effects are in line with the recently reported retardatory effects of BIS-I and H-7 on behavioural recovery after UL. They demonstrate that the increase in intrinsic excitability in MVN neurons during vestibular compensation is cerebellum dependent, and requires mGluR activation and protein phosphorylation in cerebellar cortex. Furthermore, microinjection of the glucocorticoid receptor (GR) antagonist RU38486 into the ipsi-lesional flocculus also abolished CIE in MVN neurons. Thus an important site for glucocorticoids in facilitating vestibular compensation is within the cerebellar cortex. These observations ascribe functional significance to the high levels of GR and 11-beta-HSD Type 1 expression in cerebellum.
Figures

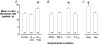
Similar articles
-
Lesion-induced plasticity in rat vestibular nucleus neurones dependent on glucocorticoid receptor activation.J Physiol. 1999 Jul 1;518(Pt 1):151-8. doi: 10.1111/j.1469-7793.1999.0151r.x. J Physiol. 1999. PMID: 10373697 Free PMC article.
-
Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurones after unilateral labyrinthectomy.J Physiol. 2000 Mar 1;523 Pt 2(Pt 2):413-24. doi: 10.1111/j.1469-7793.2000.t01-1-00413.x. J Physiol. 2000. PMID: 10699085 Free PMC article.
-
Intrinsic excitability changes in vestibular nucleus neurons after unilateral deafferentation.Brain Res. 2001 Jul 20;908(1):58-66. doi: 10.1016/s0006-8993(01)02600-2. Brain Res. 2001. PMID: 11457431
-
Molecular mechanisms of brainstem plasticity. The vestibular compensation model.Mol Neurobiol. 1991;5(2-4):355-68. doi: 10.1007/BF02935558. Mol Neurobiol. 1991. PMID: 1668392 Review.
-
Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity.Prog Neurobiol. 2005 Aug;76(6):349-92. doi: 10.1016/j.pneurobio.2005.10.002. Epub 2005 Nov 2. Prog Neurobiol. 2005. PMID: 16263204 Review.
Cited by
-
Amino acid transporter (VIAAT, VGLUT2) and chloride cotransporter (KCC1, KCC2 and NKCC1) expression in the vestibular nuclei of intact and labyrinthectomized rat.Exp Brain Res. 2007 Oct;182(4):449-58. doi: 10.1007/s00221-007-1006-0. Epub 2007 Jun 28. Exp Brain Res. 2007. PMID: 17598093
-
Expression of glycine receptors and gephyrin in rat medial vestibular nuclei and flocculi following unilateral labyrinthectomy.Int J Mol Med. 2016 Nov;38(5):1481-1489. doi: 10.3892/ijmm.2016.2753. Epub 2016 Sep 27. Int J Mol Med. 2016. PMID: 28026001 Free PMC article.
-
How Does the Central Nervous System for Posture and Locomotion Cope With Damage-Induced Neural Asymmetry?Front Syst Neurosci. 2022 Mar 3;16:828532. doi: 10.3389/fnsys.2022.828532. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35308565 Free PMC article. Review.
-
Advances in Auditory and Vestibular Medicine.Audiol Med. 2009 Dec 1;7(4):180-188. doi: 10.3109/02841860903364076. Audiol Med. 2009. PMID: 20711412 Free PMC article.
-
Responses of central vestibular neurons to sinusoidal yaw rotation in compensated macaques after unilateral labyrinthectomy.J Neurophysiol. 2013 Oct;110(8):1822-36. doi: 10.1152/jn.00365.2013. Epub 2013 Jul 17. J Neurophysiol. 2013. PMID: 23864379 Free PMC article.
References
-
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. Journal of Neurophysiology. 1999;82:1697–1709. - PubMed
-
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nature Neuroscience. 2000;3:109–111. - PubMed
-
- Azzena GB, Mameli O, Tolu E. Cerebellar contribution in compensating the vestibular function. Progress in Brain Research. 1979;50:599–606. - PubMed
-
- Babalian AL, Vidal PP. Floccular modulation of vestibuloocular pathways and cerebellum-related plasticity: An in vitro whole brain study. Journal of Neurophysiology. 2000;84:2514–2528. - PubMed
-
- Balaban CD, Freilino M, Romero GG. Protein kinase C inhibition blocks the early appearance of vestibular compensation. Brain Research. 1999;845:97–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

