Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat
- PMID: 12482904
- PMCID: PMC2290709
- DOI: 10.1113/jphysiol.2002.023408
Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat
Abstract
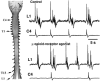
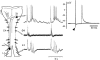
We report that after spontaneous breathing movements are stopped by administration of opioids (opioid-induced apnoea) in neonatal rats, abdominal muscles continue to contract at a rate similar to that observed during periods of ventilation. Correspondingly, in vitro bath application of a mu opioid receptor agonist suppresses the activity of the fourth cervical root (C4) supplying the diaphragm, but not the rhythmic activity of the first lumbar root (L1) innervating the abdominal muscles. This indicates the existence of opioid-resistant rhythmogenic neurones and a neuronal pathway transmitting their activity to the abdominal motoneurones. We have investigated this pathway by using a brainstem-spinal cord preparation of the neonatal rat. We identified bulbospinal neurones with a firing pattern identical to that of the L1 root. These neurones were located caudal to the obex in the vicinity of the nucleus retroambiguus. Resting potentials ranged from -49 to -40 mV (mean +/- S.D. -44.0 +/- 4.3 mV). The mean input resistance was 315.5 +/- 54.8 MOmega. The mean antidromic latency from the L1 level was 42.8 +/- 4.4 ms. Axons crossed the midline at the level of the cell body. The activity pattern of the bulbospinal neurones and the L1 root consisted of two bursts per respiratory cycle with a silent period during inspiration. This pattern is characteristic of preinspiratory neurones. We found that 11 % of the preinspiratory neurones projected to the area where the bulbospinal neurones were located. These preinspiratory neurones were found in the rostral ventrolateral medulla close (200-350 microm) to the ventral surface at the level of the rostral half of the nucleus retrofacialis. Our data suggest the operation of a disynaptic pathway from the preinspiratory neurones to the L1 motoneurones in the in vitro preparation. We propose that the same pathway is responsible for rhythmic activation of the abdominal muscles during opioid-induced apnoea in the newborn rat.
Figures
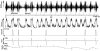
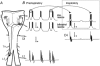


Similar articles
-
Respiration-related rhythmic activity in the rostral medulla of newborn rats.J Neurophysiol. 2006 Jul;96(1):55-61. doi: 10.1152/jn.01175.2005. Epub 2006 Feb 22. J Neurophysiol. 2006. PMID: 16495360
-
Anoxic persistence of lumbar respiratory bursts and block of lumbar locomotion in newborn rat brainstem spinal cords.J Physiol. 2007 Dec 1;585(Pt 2):507-24. doi: 10.1113/jphysiol.2007.143594. Epub 2007 Oct 11. J Physiol. 2007. PMID: 17932145 Free PMC article.
-
Biphasic effects of substance P on respiratory activity and respiration-related neurones in ventrolateral medulla in the neonatal rat brainstem in vitro.Acta Physiol Scand. 2002 Jan;174(1):67-84. doi: 10.1046/j.1365-201x.2002.00926.x. Acta Physiol Scand. 2002. PMID: 11851598
-
Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro.Br J Pharmacol. 1994 Sep;113(1):121-8. doi: 10.1111/j.1476-5381.1994.tb16183.x. Br J Pharmacol. 1994. PMID: 7812601 Free PMC article.
-
Control of abdominal muscles.Prog Neurobiol. 1998 Nov;56(4):433-506. doi: 10.1016/s0301-0082(98)00046-x. Prog Neurobiol. 1998. PMID: 9775401 Review.
Cited by
-
Neural bases for the genesis and CO2 therapy of periodic Cheyne-Stokes breathing in neonatal male connexin-36 knockout mice.Front Neurosci. 2023 Feb 8;17:1045269. doi: 10.3389/fnins.2023.1045269. eCollection 2023. Front Neurosci. 2023. PMID: 36845442 Free PMC article.
-
Induction of a parafacial rhythm generator by rhombomere 3 in the chick embryo.J Neurosci. 2004 Oct 20;24(42):9383-90. doi: 10.1523/JNEUROSCI.2408-04.2004. J Neurosci. 2004. PMID: 15496674 Free PMC article.
-
Chapter 3--networks within networks: the neuronal control of breathing.Prog Brain Res. 2011;188:31-50. doi: 10.1016/B978-0-444-53825-3.00008-5. Prog Brain Res. 2011. PMID: 21333801 Free PMC article. Review.
-
Atoh1-dependent rhombic lip neurons are required for temporal delay between independent respiratory oscillators in embryonic mice.Elife. 2014 May 14;3:e02265. doi: 10.7554/eLife.02265. Elife. 2014. PMID: 24842997 Free PMC article.
-
Bimodal Respiratory-Locomotor Neurons in the Neonatal Rat Spinal Cord.J Neurosci. 2016 Jan 20;36(3):926-37. doi: 10.1523/JNEUROSCI.1825-15.2016. J Neurosci. 2016. PMID: 26791221 Free PMC article.
References
-
- Arita H, Kogo N, Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Research. 1987;401:258–266. - PubMed
-
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Progress in Neurobiology. 1999;59:583–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

