Differential modulation of synaptic transmission by neuropeptide Y in rat neocortical neurons
- PMID: 12482942
- PMCID: PMC139280
- DOI: 10.1073/pnas.012481899
Differential modulation of synaptic transmission by neuropeptide Y in rat neocortical neurons
Abstract
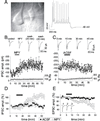
Neuropeptide Y (NPY) is widely expressed throughout the nervous system and is known to reduce excitatory (but also inhibitory) synaptic transmission in many CNS areas, leading to the proposal that it is an endogenous antiepileptic agent. In the neocortex, where NPY is present in gamma-aminobutyric acid (GABA)ergic interneurons, its effects on inhibitory and excitatory synaptic activities have not been completely explored. Here we report that NPY application elicits a long-lasting decrease in evoked excitatory postsynaptic current amplitude and a delayed, long-lasting increase in the amplitude of evoked monosynaptic inhibitory postsynaptic current (IPSC) in layer V pyramidal neurons of rat neocortex. The novel, late, NPY-mediated increase of inhibitory synaptic transmission is caused by modulation of Ca2+-dependent GABA release onto pyramidal neurons, as it was accompanied by an increase in Ca2+-dependent miniature IPSC frequency. NPY decreased evoked monosynaptic IPSCs in GABAergic interneurons, indicating that this neuropeptide has differential effects on different neuronal subtypes in the neocortex. Each of these NPY actions would decrease excitability in cortical circuits, a result that has important implications for both physiological neocortical operations as well as pathophysiological epileptiform activities.
Figures

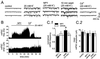

References
-
- Wahlestedt C. & Reis, D. J. (1993) Annu. Rev. Pharmacol. Toxicol. 33, 309-352. - PubMed
-
- Balasubramaniam A. A. (1997) Peptides 18, 445-457. - PubMed
-
- Blomqvist A. G. & Herzog, H. (1997) Trends Neurosci. 20, 294-298. - PubMed
-
- Michel M. C., Beck-Sickinger, A., Cox, H., Doods, H. N., Herzog, H., Larhammar, D., Quirion, R., Schwartz, T. & Westfall, T. (1998) Pharmacol. Rev. 50, 143-150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

