A CADASIL-mutated Notch 3 receptor exhibits impaired intracellular trafficking and maturation but normal ligand-induced signaling
- PMID: 12482954
- PMCID: PMC139279
- DOI: 10.1073/pnas.252624099
A CADASIL-mutated Notch 3 receptor exhibits impaired intracellular trafficking and maturation but normal ligand-induced signaling
Abstract
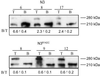
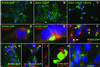
Notch receptors are single transmembrane receptors that contain a large number of epidermal growth factor-like repeats (EGF repeats) in their extracellular domains. Mutations in the EGF repeats of the human Notch 3 receptor lead to the vascular dementia disease Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL). The vast majority of CADASIL mutations are missense mutations removing or inserting cysteine residues in the EGF repeats, but it is not yet clear whether these mutations primarily affect receptor trafficking, maturation, andor signaling. To address this issue, we have generated and analyzed stable cell lines expressing either wild-type murine Notch 3 (mNotch 3) or the mutant mNotch 3(R142C), which corresponds to the prevalent CADASIL form of Notch 3, Notch 3(R141C) in humans. We find that a lower proportion of mNotch 3(R142C) is expressed in the site 1-cleaved configuration, and that reduced amounts of mNotch 3(R142C) appear at the cell surface, as compared with wild-type mNotch 3. This observation is accompanied by a higher propensity for mNotch 3(R142C) to form intracellular aggregates, which may be a result of increased accumulation or slowed transport in the secretory pathway. In contrast to the impaired cell surface expression, mNotch 3(R142C) signals equally well in response to Delta 1 and Jagged 1 as wild-type mNotch 3. Taken together, these data suggest that trafficking and localization rather than signaling of mNotch 3 are affected in mNotch 3(R142C).
Figures

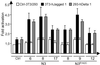

Similar articles
-
CADASIL-associated Notch3 mutations have differential effects both on ligand binding and ligand-induced Notch3 receptor signaling through RBP-Jk.Exp Cell Res. 2004 Oct 1;299(2):454-64. doi: 10.1016/j.yexcr.2004.06.004. Exp Cell Res. 2004. PMID: 15350543
-
Pathogenic mutations associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy differently affect Jagged1 binding and Notch3 activity via the RBP/JK signaling Pathway.Am J Hum Genet. 2004 Feb;74(2):338-47. doi: 10.1086/381506. Epub 2004 Jan 8. Am J Hum Genet. 2004. PMID: 14714274 Free PMC article.
-
Mice carrying a R142C Notch 3 knock-in mutation do not develop a CADASIL-like phenotype.Genesis. 2005 Jan;41(1):13-22. doi: 10.1002/gene.20091. Genesis. 2005. PMID: 15645445
-
CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia.Brain Pathol. 2002 Jul;12(3):371-84. doi: 10.1111/j.1750-3639.2002.tb00451.x. Brain Pathol. 2002. PMID: 12146805 Free PMC article. Review.
-
Notch signaling in vascular development.Arterioscler Thromb Vasc Biol. 2003 Apr 1;23(4):543-53. doi: 10.1161/01.ATV.0000060892.81529.8F. Epub 2003 Feb 13. Arterioscler Thromb Vasc Biol. 2003. PMID: 12615665 Review.
Cited by
-
Signaling required for blood vessel maintenance: molecular basis and pathological manifestations.Int J Vasc Med. 2012;2012:293641. doi: 10.1155/2012/293641. Epub 2011 Dec 6. Int J Vasc Med. 2012. PMID: 22187650 Free PMC article.
-
CADASIL: experimental insights from animal models.Stroke. 2010 Oct;41(10 Suppl):S129-34. doi: 10.1161/STROKEAHA.110.595207. Stroke. 2010. PMID: 20876488 Free PMC article. Review.
-
Signaling pathways and molecular mechanisms involved in the onset and progression of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL); a focus on Notch3 signaling.J Headache Pain. 2025 Apr 29;26(1):96. doi: 10.1186/s10194-025-02025-z. J Headache Pain. 2025. PMID: 40301727 Free PMC article. Review.
-
Biochemical characterization and cellular effects of CADASIL mutants of NOTCH3.PLoS One. 2012;7(9):e44964. doi: 10.1371/journal.pone.0044964. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028706 Free PMC article.
-
Stroke-related translational research.Arch Neurol. 2011 Sep;68(9):1110-23. doi: 10.1001/archneurol.2011.99. Epub 2011 May 9. Arch Neurol. 2011. PMID: 21555605 Free PMC article. Review.
References
-
- Artavanis-Tsakonas S., Rand, M. D. & Lake, R. J. (1999) Science 284, 770-776. - PubMed
-
- Brou C., Logeat, F., Gupta, N., Bessia, C., LeBail, O., Doedens, J. R., Cumano, A., Roux, P., Black, R. A. & Israel, A. (2000) Mol. Cell 5, 207-216. - PubMed
-
- Mumm J. S., Schroeter, E. H., Saxena, M. T., Griesemer, A., Tian, X., Pan, D. J., Ray, W. J. & Kopan, R. (2000) Mol. Cell 2, 197-206. - PubMed
-
- De Strooper B., Annaert, W., Cupers, P., Saftig, P., Craessaerts, K., Mumm, J. S., Schroeter, E. H., Schrijvers, V., Wolfe, M. S., Ray, W. J., Goate, A. & Kopan, R. (1999) Nature 398, 518-522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

