Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10
- PMID: 12482956
- PMCID: PMC140663
- DOI: 10.1128/MCB.23.1.1-13.2003
Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10
Abstract
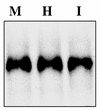
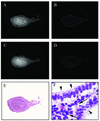
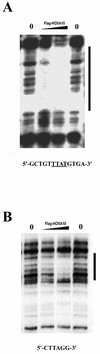
HOXA10 is necessary for mammalian reproduction; however, its transcriptional targets are not completely defined. EMX2, a divergent homeobox gene, is necessary for urogenital tract development. In these studies we identify and characterize the regulation of EMX2 by HOXA10. By using Northern analysis and in situ hybridization, we found that EMX2 is expressed in the adult urogenital tract in an inverse temporal pattern from HOXA10, suggestive of a negative regulatory relationship. Constitutive expression of HOXA10 diminished EMX2 mRNA, whereas blocking HOXA10 through the use of antisense resulted in high EMX2 mRNA expression. Deletional analysis of the EMX2 5' regulatory region revealed that a 150-bp element mediated transcriptional repression when cotransfected with pcDNA3.1/HOXA10 in transient-transfection assays. Binding of HOXA10 protein to this element was demonstrated by electrophoretic mobility shift assay and further localized to a consensus HOXA10 binding site within this element by DNase I footprinting. Site-directed mutagenesis abolished binding, as well as the negative transcriptional regulation. Transcriptional activation of empty spiracles, the Drosophila ortholog of EMX2, by Abdominal-B (HOXA10 ortholog) has been previously demonstrated. These findings demonstrate conservation of the transcription factor-target gene relationship, although the direction of regulation is reversed with possible evolutionary implications.
Figures






References
-
- Akam, M. 1989. Hox and HOM: homologous gene clusters in insects and vertebrates. Cell 57:347-349. - PubMed
-
- Awgulewitsch, A., and D. Jacobs. 1992. Deformed autoregulatory element from Drosophila functions in a conserved manner in transgenic mice. Nature 358:341-344. - PubMed
-
- Bagot, C. N., H. J. Kliman, and H. S. Taylor. 2001. Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev. Dyn. 222:538-544. - PubMed
-
- Bagot, C. N., P. J. Troy, and H. S. Taylor. 2000. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther. 7:1378-1384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
