Iron status in mice carrying a targeted disruption of lactoferrin
- PMID: 12482971
- PMCID: PMC140657
- DOI: 10.1128/MCB.23.1.178-185.2003
Iron status in mice carrying a targeted disruption of lactoferrin
Abstract
Lactoferrin is a member of the transferrin family of iron-binding glycoproteins present in milk, mucosal secretions, and the secondary granules of neutrophils. While several physiological functions have been proposed for lactoferrin, including the regulation of intestinal iron uptake, the exact function of this protein in vivo remains to be established. To directly assess the physiological functions of lactoferrin, we have generated lactoferrin knockout (LFKO(-/-)) mice by homologous gene targeting. LFKO(-/-) mice are viable and fertile, develop normally, and display no overt abnormalities. A comparison of the iron status of suckling offspring from LFKO(-/-) intercrosses and from wild-type (WT) intercrosses showed that lactoferrin is not essential for iron delivery during the postnatal period. Further, analysis of adult mice on a basal or a high-iron diet revealed no differences in transferrin saturation or tissue iron stores between WT and LFKO(-/-) mice on either diet, although the serum iron levels were slightly elevated in LFKO-/- mice on the basal diet. Consistent with the relatively normal iron status, in situ hybridization analysis demonstrated that lactoferrin is not expressed in the postnatal or adult intestine. Collectively, these results support the conclusion that lactoferrin does not play a major role in the regulation of iron homeostasis.
Figures
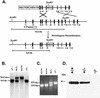
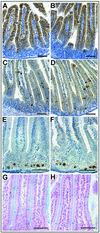

References
-
- Anderson, B. F., H. M. Baker, G. E. Norris, D. W. Rice, and E. N. Baker. 1989. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J. Mol. Biol. 209:711-734. - PubMed
-
- Andrews, N. C. 2000. Iron homeostasis: insights from genetics and animal models. Nat. Rev. Genet. 1:208-217. - PubMed
-
- Bailey, S., R. W. Evans, R. C. Garratt, B. Gorinsky, S. Hasnain, C. Horsburgh, H. Jhoti, P. F. Lindley, A. Mydin, R. Sarra, et al. 1988. Molecular structure of serum transferrin at 3.3-A resolution. Biochemistry 27:5804-5812. - PubMed
-
- Baranov, V. S., A. L. Schwartzman, V. N. Gorbunova, V. S. Gaitskhoki, N. B. Rubtsov, N. A. Timchenko, and S. A. Neifakh. 1987. Chromosomal localization of ceruloplasmin and transferrin genes in laboratory rats, mice and in man by hybridization with specific DNA probes. Chromosoma 96:60-66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
