A novel domain within the DEAD-box protein DP103 is essential for transcriptional repression and helicase activity
- PMID: 12482992
- PMCID: PMC140651
- DOI: 10.1128/MCB.23.1.414-423.2003
A novel domain within the DEAD-box protein DP103 is essential for transcriptional repression and helicase activity
Abstract
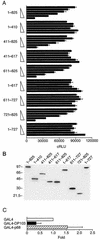
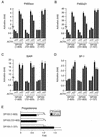
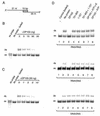
Members of the DEAD-box family of helicases, distinguished by a core characteristic sequence of Asp-Glu-Ala-Asp, are expressed in a wide range of prokaryotes and eukaryotes and exhibit diverse cellular functions, including DNA transcription, recombination and repair, RNA processing, translation, and posttranslational regulation. Although ubiquitous, the function of most DEAD-box proteins is unknown. We and others have recently cloned DP103, which harbors conserved DEAD-box, helicase, and ATPase domains in its N terminus. DP103 (also termed Gemin3 and DDX20) interacts with SF-1, SMN, EBNA2, and EBNA3C in mammalian cells. Here we demonstrate that a discrete domain within the nonconserved C-terminal region of DP103 directly interacts with SF-1. This domain exhibits an autonomous repression function and is necessary and sufficient for repressing the transcriptional activity of SF-1. Furthermore, intact DP103 exhibits helicase activity. Importantly, the C-terminal domain is obligatory but not sufficient for this unwinding activity of DP103. Together, our results support a novel paradigm for transcriptional repression and demonstrate the bifunctional role of the C-terminal domain of DP103.
Figures

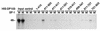

References
-
- Campbell, L., K. M. Hunter, P. Mohaghegh, J. M. Tinsley, M. A. Brasch, and K. E. Davies. 2000. Direct interaction of Smn with dp103, a putative RNA helicase: a role for Smn in transcription regulation? Hum. Mol. Genet. 9:1093-1100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
