Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle
- PMID: 12485990
- PMCID: PMC139109
- DOI: 10.1093/emboj/cdf689
Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle
Abstract
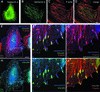
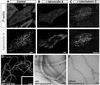
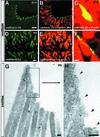
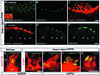
Deaf-blindness in three distinct genetic forms of Usher type I syndrome (USH1) is caused by defects in myosin VIIa, harmonin and cadherin 23. Despite being critical for hearing, the functions of these proteins in the inner ear remain elusive. Here we show that harmonin, a PDZ domain-containing protein, and cadherin 23 are both present in the growing stereocilia and that they bind to each other. Moreover, we demonstrate that harmonin b is an F-actin-bundling protein, which is thus likely to anchor cadherin 23 to the stereocilia microfilaments, thereby identifying a novel anchorage mode of the cadherins to the actin cytoskeleton. Moreover, harmonin b interacts directly with myosin VIIa, and is absent from the disorganized hair bundles of myosin VIIa mutant mice, suggesting that myosin VIIa conveys harmonin b along the actin core of the developing stereocilia. We propose that the shaping of the hair bundle relies on a functional unit composed of myosin VIIa, harmonin b and cadherin 23 that is essential to ensure the cohesion of the stereocilia.
Figures



References
-
- Alagramam K.N. et al. (2001a) Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum. Mol. Genet., 10, 1709–1718. - PubMed
-
- Alagramam K.N., Murcia,C.L., Kwon,H.Y., Pawlowski,K.S., Wright,C.G. and Woychik,R.P. (2001b) The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat. Genet., 27, 99–102. - PubMed
-
- Angst B.D., Marcozzi,C. and Magee,A.I. (2001) The cadherin superfamily. J. Cell Sci., 114, 625–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

