In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation
- PMID: 12486003
- PMCID: PMC139102
- DOI: 10.1093/emboj/cdf682
In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation
Abstract
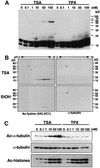
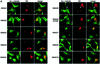
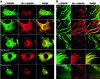
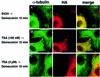
Trichostatin A (TSA) inhibits all histone deacetylases (HDACs) of both class I and II, whereas trapoxin (TPX) cannot inhibit HDAC6, a cytoplasmic member of class II HDACs. We took advantage of this differential sensitivity of HDAC6 to TSA and TPX to identify its substrates. Using this approach, alpha-tubulin was identified as an HDAC6 substrate. HDAC6 deacetylated alpha-tubulin both in vivo and in vitro. Our investigations suggest that HDAC6 controls the stability of a dynamic pool of microtubules. Indeed, we found that highly acetylated microtubules observed after TSA treatment exhibited delayed drug-induced depolymerization and that HDAC6 overexpression prompted their induced depolymerization. Depolymerized tubulin was rapidly deacetylated in vivo, whereas tubulin acetylation occurred only after polymerization. We therefore suggest that acetylation and deacetylation are coupled to the microtubule turnover and that HDAC6 plays a key regulatory role in the stability of the dynamic microtubules.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

