Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures
- PMID: 12486012
- PMCID: PMC139098
- DOI: 10.1093/emboj/cdf678
Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures
Abstract
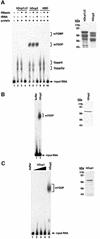
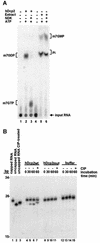
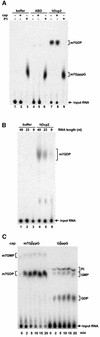
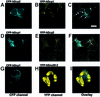
We have cloned cDNAs for the human homologues of the yeast Dcp1 and Dcp2 factors involved in the major (5'-3') and NMD mRNA decay pathways. While yeast Dcp1 has been reported to be the decapping enzyme, we show that recombinant human Dcp2 (hDcp2) is enzymatically active. Dcp2 activity appears evolutionarily conserved. Mutational and biochemical analyses indicate that the hDcp2 MutT/Nudix domain mediates this activity. hDcp2 generates m7GDP and 5'-phosphorylated mRNAs that are 5'-3' exonuclease substrates. Corresponding decay intermediates are present in human cells showing the relevance of this activity. hDcp1 and hDcp2 co-localize in cell cytoplasm, consistent with a role in mRNA decay. Interestingly, these two proteins show a non-uniform distribution, accumulating in specific foci.
Figures



References
-
- Bai R.Y., Koester,C., Ouyang,T., Hahn,S.A., Hammerschmidt,M., Peschel,C. and Duyster,J. (2002) SMIF, a Smad4-interacting protein that functions as a co-activator in TGFβ signalling. Nat. Cell Biol., 4, 181–190. - PubMed
-
- Beelman C.A., Stevens,A., Caponigro,G., LaGrandeur,T.E., Hatfield,L., Fortner,D.M. and Parker,R. (1996) An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature, 382, 642–646. - PubMed
-
- Bessman M.J., Frick,D.N. and O’Handley,S.F. (1996) The MutT proteins or ‘Nudix’ hydrolases, a family of versatile, widely distributed, ‘housecleaning’ enzymes. J. Biol. Chem., 271, 25059–25062. - PubMed
-
- Bousquet-Antonelli C., Presutti,C. and Tollervey,D. (2000) Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell, 102, 765–775. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

