The SecB chaperone is bifunctional in Serratia marcescens: SecB is involved in the Sec pathway and required for HasA secretion by the ABC transporter
- PMID: 12486043
- PMCID: PMC141835
- DOI: 10.1128/JB.185.1.80-88.2003
The SecB chaperone is bifunctional in Serratia marcescens: SecB is involved in the Sec pathway and required for HasA secretion by the ABC transporter
Abstract
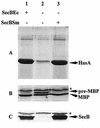
HasA is the secreted hemophore of the heme acquisition system (Has) of Serratia marcescens. It is secreted by a specific ABC transporter apparatus composed of three proteins: HasD, an inner membrane ABC protein; HasE, another inner membrane protein; and HasF, a TolC homolog. Except for HasF, the structural genes of the Has system are encoded by an iron-regulated operon. In previous studies, this secretion system has been reconstituted in Escherichia coli, where it requires the presence of the SecB chaperone, the Sec pathway-dedicated chaperone. We cloned and inactivated the secB gene from S. marcescens. We show that S. marcescens SecB is 93% identical to E. coli SecB and complements the secretion defects of a secB mutant of E. coli for both the Sec and ABC pathways of HasA secretion. In S. marcescens, SecB inactivation affects translocation by the Sec pathway and abolishes HasA secretion. This demonstrates that S. marcescens SecB is the genuine chaperone for HasA secretion in S. marcescens. These results also demonstrate that S. marcescens SecB is bifunctional, as it is involved in two separate secretion pathways. We investigated the effects of secB point mutations in the reconstituted HasA secretion pathway by comparing the translocation of a Sec substrate in various mutants. Two different patterns of SecB residue effects were observed, suggesting that SecB functions may differ for the Sec and ABC pathways.
Figures




Similar articles
-
The N terminus of the HasA protein and the SecB chaperone cooperate in the efficient targeting and secretion of HasA via the ATP-binding cassette transporter.J Biol Chem. 2002 Feb 22;277(8):6726-32. doi: 10.1074/jbc.M108632200. Epub 2001 Nov 6. J Biol Chem. 2002. PMID: 11698405
-
Cloning of the Serratia marcescens hasF gene encoding the Has ABC exporter outer membrane component: a TolC analogue.Mol Microbiol. 1996 Oct;22(2):265-73. doi: 10.1046/j.1365-2958.1996.00103.x. Mol Microbiol. 1996. PMID: 8930911
-
The SecB chaperone is involved in the secretion of the Serratia marcescens HasA protein through an ABC transporter.EMBO J. 1998 Feb 16;17(4):936-44. doi: 10.1093/emboj/17.4.936. EMBO J. 1998. PMID: 9463372 Free PMC article.
-
Protein secretion by Gram-negative bacterial ABC exporters--a review.Gene. 1997 Jun 11;192(1):7-11. doi: 10.1016/s0378-1119(96)00829-3. Gene. 1997. PMID: 9224868 Review.
-
Protein secretion by gram-negative bacterial ABC exporters.Folia Microbiol (Praha). 1997;42(3):179-83. doi: 10.1007/BF02818975. Folia Microbiol (Praha). 1997. PMID: 9246759 Review.
Cited by
-
Characterization of a heme-regulated non-coding RNA encoded by the prrF locus of Pseudomonas aeruginosa.PLoS One. 2010 Apr 8;5(4):e9930. doi: 10.1371/journal.pone.0009930. PLoS One. 2010. PMID: 20386693 Free PMC article.
-
Proteomic profiling of Serratia marcescens by high-resolution mass spectrometry.Bioimpacts. 2020;10(2):123-135. doi: 10.34172/bi.2020.15. Epub 2020 Mar 26. Bioimpacts. 2020. PMID: 32363156 Free PMC article.
-
Multiple signals direct the assembly and function of a type 1 secretion system.J Bacteriol. 2010 Aug;192(15):3861-9. doi: 10.1128/JB.00178-10. Epub 2010 Apr 23. J Bacteriol. 2010. PMID: 20418390 Free PMC article.
-
The Sec System: Protein Export in Escherichia coli.EcoSal Plus. 2017 Nov;7(2):10.1128/ecosalplus.ESP-0002-2017. doi: 10.1128/ecosalplus.ESP-0002-2017. EcoSal Plus. 2017. PMID: 29165233 Free PMC article. Review.
-
Mutation of crp mediates Serratia marcescens serralysin and global secreted protein production.Res Microbiol. 2013 Jan;164(1):38-45. doi: 10.1016/j.resmic.2012.10.006. Epub 2012 Oct 13. Res Microbiol. 2013. PMID: 23072819 Free PMC article.
References
-
- Binet, R., S. Letoffe, J. M. Ghigo, P. Delepelaire, and C. Wandersman. 1997. Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene 192:7-11. - PubMed
-
- Binet, R., and C. Wandersman. 1996. Cloning of the Serratia marcescens hasF gene encoding the Has ABC exporter outer membrane component: a TolC analogue. Mol. Microbiol. 22:265-273. - PubMed
-
- Blondelet-Rouault, M. H., J. Weiser, A. Lebrihi, P. Branny, and J. L. Pernodet. 1997. Antibiotic resistance gene cassettes derived from the omega interposon for use in E. coli and Streptomyces. Gene 190:315-317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

