Metal ion dependence of recombinant Escherichia coli allantoinase
- PMID: 12486048
- PMCID: PMC141845
- DOI: 10.1128/JB.185.1.126-134.2003
Metal ion dependence of recombinant Escherichia coli allantoinase
Abstract
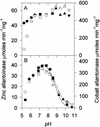
Allantoinase is a suspected dinuclear metalloenzyme that catalyzes the hydrolytic cleavage of the five-member ring of allantoin (5-ureidohydantoin) to form allantoic acid. Recombinant Escherichia coli allantoinase purified from overproducing cultures amended with 2.5 mM zinc, 1 mM cobalt, or 1 mM nickel ions was found to possess approximately 1.4 Zn, 0.0 Co, 0.0 Ni, and 0.4 Fe; 0.1 Zn, 1.0 Co, 0.0 Ni, and 0.2 Fe; and 0.0 Zn, 0.0 Co, 0.6 Ni, and 0.1 Fe per subunit, respectively, whereas protein obtained from nonamended cultures contains near stoichiometric levels of iron. We conclude that allantoinase is incompletely activated in the recombinant cells, perhaps due to an insufficiency of a needed accessory protein. Enzyme isolated from nonsupplemented cultures possesses very low activity (k(cat) = 34.7 min(-1)) compared to the zinc-, cobalt-, and nickel-containing forms of allantoinase (k(cat) values of 5,000 and 28,200 min(-1) and 200 min(-1), respectively). These rates and corresponding K(m) values (17.0, 19.5, and 80 mM, respectively) are significantly greater than those that have been reported previously. Absorbance spectroscopy of the cobalt species reveals a band centered at 570 nm consistent with five-coordinate geometry. Dithiothreitol is a competitive inhibitor of the enzyme, with significant K(i) differences for the zinc and cobalt species (237 and 795 micro M, respectively). Circular dichroism spectroscopy revealed that the zinc enzyme utilizes only the S isomer of allantoin, whereas the cobalt allantoinase prefers the S isomer, but also hydrolyzes the R isomer at about 1/10 the rate. This is the first report for metal content of allantoinase from any source.
Figures




Similar articles
-
Biochemical characterization of allantoinase from Escherichia coli BL21.Protein J. 2011 Aug;30(6):384-94. doi: 10.1007/s10930-011-9343-z. Protein J. 2011. PMID: 21739308
-
Crystal structure of metal-dependent allantoinase from Escherichia coli.J Mol Biol. 2009 Apr 17;387(5):1067-74. doi: 10.1016/j.jmb.2009.02.041. Epub 2009 Feb 24. J Mol Biol. 2009. PMID: 19248789
-
Biochemical and mutational studies of allantoinase from Bacillus licheniformis CECT 20T.Biochimie. 2014 Apr;99:178-88. doi: 10.1016/j.biochi.2013.12.002. Epub 2013 Dec 12. Biochimie. 2014. PMID: 24333989
-
Functional expression and characterization of the two cyclic amidohydrolase enzymes, allantoinase and a novel phenylhydantoinase, from Escherichia coli.J Bacteriol. 2000 Dec;182(24):7021-8. doi: 10.1128/JB.182.24.7021-7028.2000. J Bacteriol. 2000. PMID: 11092864 Free PMC article.
-
Purification, substrate range, and metal center of AtzC: the N-isopropylammelide aminohydrolase involved in bacterial atrazine metabolism.J Bacteriol. 2002 Oct;184(19):5376-84. doi: 10.1128/JB.184.19.5376-5384.2002. J Bacteriol. 2002. PMID: 12218024 Free PMC article.
Cited by
-
Functional characterization of allantoinase genes from Arabidopsis and a nonureide-type legume black locust.Plant Physiol. 2004 Mar;134(3):1039-49. doi: 10.1104/pp.103.034637. Epub 2004 Feb 19. Plant Physiol. 2004. PMID: 14976234 Free PMC article.
-
Ureidoglycolate hydrolase, amidohydrolase, lyase: how errors in biological databases are incorporated in scientific papers and vice versa.Database (Oxford). 2013 Oct 9;2013:bat071. doi: 10.1093/database/bat071. Print 2013. Database (Oxford). 2013. PMID: 24107613 Free PMC article.
-
Fructose-1,6-bisphosphate aldolase (class II) is the primary site of nickel toxicity in Escherichia coli.Mol Microbiol. 2011 Dec;82(5):1291-300. doi: 10.1111/j.1365-2958.2011.07891.x. Epub 2011 Nov 8. Mol Microbiol. 2011. PMID: 22014167 Free PMC article.
-
Characterization of the structure and function of Klebsiella pneumoniae allantoin racemase.J Mol Biol. 2011 Jul 15;410(3):447-60. doi: 10.1016/j.jmb.2011.05.016. Epub 2011 May 17. J Mol Biol. 2011. PMID: 21616082 Free PMC article.
-
Co(II)-substituted Haemophilus influenzae β-carbonic anhydrase: spectral evidence for allosteric regulation by pH and bicarbonate ion.Arch Biochem Biophys. 2011 Jul;511(1-2):80-7. doi: 10.1016/j.abb.2011.04.013. Epub 2011 Apr 22. Arch Biochem Biophys. 2011. PMID: 21531201 Free PMC article.
References
-
- Abendroth, J., K. Niefind, O. May, M. Siemann, C. Syldatk, and D. Schomburg. 2002. The structure of L-hydantoinase from Arthrobacter aurescens leads to an understanding of dihydropyrimidinase substrate and enantio specificity. Biochemistry 41:8589-8597. - PubMed
-
- Abendroth, J., K. Niefind, and D. Schomburg. 2002. X-ray structure of a dihydropyrimidinase from Thermus sp. at 1.3 Å resolution. J. Mol. Biol. 320:143-156. - PubMed
-
- Ausubel, F. M. 1994. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
-
- Benning, M. M., J. M. Kuo, F. M. Raushel, and H. M. Holden. 1995. 3-Dimensional structure of the binuclear metal center of phosphotriesterase. Biochemistry 34:7973-7978. - PubMed
-
- Bertini, I., and C. Luchinat. 1994. The reaction pathways of zinc enzymes and related biological catalysts, p. 37-106. In I. Bertini, H. B. Gray, S. J. Lippard, and J. S. Valentine (ed.), Bioinorganic chemistry. University Science Books, Mill Valley, Calif.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

