Interdomain linkers of homologous response regulators determine their mechanism of action
- PMID: 12486069
- PMCID: PMC141822
- DOI: 10.1128/JB.185.1.317-324.2003
Interdomain linkers of homologous response regulators determine their mechanism of action
Abstract
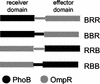
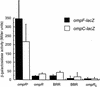
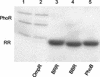
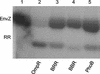
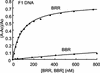
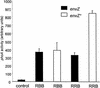
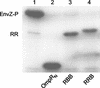
OmpR and PhoB are response regulators that contain an N-terminal phosphorylation domain and a C-terminal DNA binding effector domain connected by a flexible interdomain linker. Phosphorylation of the N terminus results in an increase in affinity for specific DNA and the subsequent regulation of gene expression. Despite their sequence and structural similarity, OmpR and PhoB employ different mechanisms to regulate their effector domains. Phosphorylation of OmpR in the N terminus stimulates the DNA binding affinity of the C terminus, whereas phosphorylation of the PhoB N terminus relieves inhibition of the C terminus, enabling it to bind to DNA. Chimeras between OmpR and PhoB containing either interdomain linker were constructed to explore the basis of the differences in their activation mechanisms. Our results indicate that effector domain regulation by either N terminus requires its cognate interdomain linker. In addition, our findings suggest that the isolated C terminus of OmpR is not sufficient for a productive interaction with RNA polymerase.
Figures
References
-
- Aiba, H., T. Mizuno, and S. Mizushima. 1989. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J. Biol. Chem. 264:8563-8567. - PubMed
-
- Aiba, H., F. Nakasai, S. Mizushima, and T. Mizuno. 1989. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, results in stimulation of its DNA-binding ability. J. Biochem. (Tokyo) 106:5-7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

