Membrane topology of the ZntB efflux system of Salmonella enterica serovar Typhimurium
- PMID: 12486076
- PMCID: PMC141924
- DOI: 10.1128/JB.185.1.374-376.2003
Membrane topology of the ZntB efflux system of Salmonella enterica serovar Typhimurium
Abstract
The membrane topology of the ZntB Zn(2+) transport protein of Salmonella enterica serovar Typhimurium was determined by constructing deletion derivatives of the protein and genetically fusing them to blaM or lacZ cassettes. The enzymatic activities of the hybrid proteins indicate that ZntB is a bitopic integral membrane protein consisting largely of two independent domains. The first 266 amino acids form a large, highly charged domain within the cytoplasm, while the remaining 61 residues form a small membrane domain containing two membrane-spanning segments. The overall orientation towards the cytoplasm is consistent with the ability of ZntB to facilitate zinc efflux.
Figures
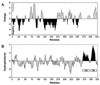
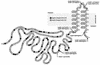
References
-
- Argos, P., and J. K. Rao. 1986. Prediction of protein structure. Methods Enzymol. 130:185-207. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. A. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, N.Y.
-
- Broome-Smith, J. K., M. Tadayyon, and Y. Zhang. 1990. Beta-lactamase as a probe of membrane protein assembly and protein export. Mol. Microbiol. 4:1637-1644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

