CD1-mediated gamma/delta T cell maturation of dendritic cells
- PMID: 12486100
- PMCID: PMC2196072
- DOI: 10.1084/jem.20021515
CD1-mediated gamma/delta T cell maturation of dendritic cells
Abstract
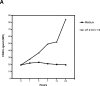
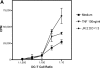
Immature myeloid dendritic cells (DCs) express only low levels of major histocompatibility complex (MHC) class II but express high levels of CD1 a, b, and c antigen-presenting molecules at the cell surface. As Vdelta1+ gamma/delta T cells are the main tissue subset of gamma/delta T cells and they are known to recognize CD1c in the absence of specific foreign antigen recognition, we examined the possible interaction of these T cells with immature DCs. We show that CD1-restricted gamma/delta T cells can mediate the maturation of DCs. DC maturation required cell-cell contact and could be blocked by antibodies against CD1c. The maturation process was partially mediated by tumor necrosis factor alpha. Importantly, immature DCs matured in the presence of lipopolysaccharide and CD1-restricted gamma/delta T cells produced bioactive interleukin-12p70. In addition, these DCs were able to efficiently present peptide antigens to naive CD4+ T cells. CD1-restricted gamma/delta T cell recognition of immature DCs provides the human immune system with the capacity to rapidly generate a pool of mature DCs early during microbial invasion. This may be an important source of critical host signals for T helper type 1 polarization of antigen-specific naive T cells and the subsequent adaptive immune response.
Figures









Similar articles
-
CD1 molecules efficiently present antigen in immature dendritic cells and traffic independently of MHC class II during dendritic cell maturation.J Immunol. 2002 Nov 1;169(9):4770-7. doi: 10.4049/jimmunol.169.9.4770. J Immunol. 2002. PMID: 12391186
-
Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity.J Exp Med. 2000 Mar 20;191(6):937-48. doi: 10.1084/jem.191.6.937. J Exp Med. 2000. PMID: 10727456 Free PMC article.
-
Dendritic cells cultured in anti-CD40 antibody-immobilized plates elicit a highly efficient peptide-specific T-cell response.J Immunother. 2002 Mar-Apr;25(2):176-84. doi: 10.1097/00002371-200203000-00005. J Immunother. 2002. PMID: 12074047
-
CD1 antigen presentation and infectious disease.Contrib Microbiol. 2003;10:164-82. doi: 10.1159/000068136. Contrib Microbiol. 2003. PMID: 12530326 Review.
-
Gamma delta T cells and dendritic cells: close partners and biological adjuvants for new therapies.Curr Mol Med. 2007 Nov;7(7):658-73. doi: 10.2174/156652407782564345. Curr Mol Med. 2007. PMID: 18045144 Review.
Cited by
-
From Host Defense to Metabolic Signatures: Unveiling the Role of γδ T Cells in Bacterial Infections.Biomolecules. 2024 Feb 15;14(2):225. doi: 10.3390/biom14020225. Biomolecules. 2024. PMID: 38397462 Free PMC article. Review.
-
Activation and selective IL-17 response of human Vγ9Vδ2 T lymphocytes by TLR-activated plasmacytoid dendritic cells.Oncotarget. 2016 Sep 20;7(38):60896-60905. doi: 10.18632/oncotarget.11755. Oncotarget. 2016. PMID: 27590513 Free PMC article.
-
gammadelta T lymphocytes-selectable cells within the innate system?J Clin Immunol. 2007 Mar;27(2):133-44. doi: 10.1007/s10875-007-9077-z. Epub 2007 Feb 14. J Clin Immunol. 2007. PMID: 17333410 Review.
-
Bone Marrow-Resident Vδ1 T Cells Co-express TIGIT With PD-1, TIM-3 or CD39 in AML and Myeloma.Front Med (Lausanne). 2021 Nov 8;8:763773. doi: 10.3389/fmed.2021.763773. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34820398 Free PMC article.
-
Intra-articular CD1c-expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of T cell-attracting chemokines and spontaneously induce Th1, Th17 and Th2 cell activity.Arthritis Res Ther. 2013 Oct 20;15(5):R155. doi: 10.1186/ar4338. Arthritis Res Ther. 2013. PMID: 24286358 Free PMC article.
References
-
- Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. - PubMed
-
- Steinman, R.M., K. Inaba, S. Turley, P. Pierre, and I. Mellman. 1999. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum. Immunol. 60:562–567. - PubMed
-
- Lanzavecchia, A. 1999. Dendritic cell maturation and generation of immune responses. Haematologica 84 Suppl EHA-4:23-25. - PubMed
-
- Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1:311–316. - PubMed
-
- Shreedhar, V., A.M. Moodycliffe, S.E. Ullrich, C. Bucana, M.L. Kripke, and L. Flores-Romo. 1999. Dendritic cells require T cells for functional maturation in vivo. Immunity. 11:625–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

