Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity
- PMID: 12486102
- PMCID: PMC2196073
- DOI: 10.1084/jem.20020515
Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity
Abstract
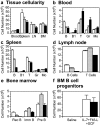
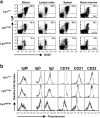
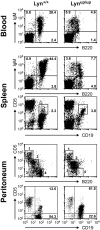
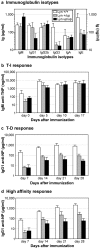
Genetic ablation of the Lyn tyrosine kinase has revealed unique inhibitory roles in B lymphocyte signaling. We now report the consequences of sustained activation of Lyn in vivo using a targeted gain-of-function mutation (Lyn(up/up) mice). Lyn(up/up) mice have reduced numbers of conventional B lymphocytes, down-regulated surface immunoglobulin M and costimulatory molecules, and elevated numbers of B1a B cells. Lyn(up/up) B cells are characterized by the constitutive phosphorylation of negative regulators of B cell antigen receptor (BCR) signaling including CD22, SHP-1, and SHIP-1, and display attributes of lymphocytes rendered tolerant by constitutive engagement of the antigen receptor. However, exaggerated positive signaling is also apparent as evidenced by the constitutive phosphorylation of Syk and phospholipase Cgamma2 in resting Lyn(up/up) B cells. Similarly, Lyn(up/up) B cells show a heightened calcium flux in response to BCR stimulation. Surprisingly, Lyn(up/up) mice develop circulating autoreactive antibodies and lethal autoimmune glomerulonephritis, suggesting that enhanced positive signaling eventually overrides constitutive negative signaling. These studies highlight the difficulty in maintaining tolerance in the face of chronic stimulation and emphasize the pivotal role of Lyn in B cell signaling.
Figures


References
-
- Healy, J.I., and C.C. Goodnow. 1998. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 16:645–670. - PubMed
-
- O'Keefe, T.L., G.T. Williams, S.L. Davies, and M.S. Neuberger. 1996. Hyperresponsive B cells in CD22-deficient mice. Science. 274:798–801. - PubMed
-
- Sato, S., A.S. Miller, M. Inaoki, C.B. Bock, P.J. Jansen, M.L. Tang, and T.F. Tedder. 1996. CD22 is both a positive and negative regulator of B-lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 5:551–562. - PubMed
-
- Otipoby, K.L., K.B. Andersson, K.E. Draves, S.J. Klaus, A.G. Garr, J.D. Kerner, R.M. Perlmutter, C.-L. Law, and E.A. Clark. 1996. CD22 regulates thymus independent responses and the lifespan of B cells. Nature. 384:634–637. - PubMed
-
- Nitschke, L., R. Carsetti, B. Ocker, G. Kohler, and M.C. Lamers. 1997. CD22 is a negative regulator of B cell receptor signaling. Curr. Biol. 7:133–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

