Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling
- PMID: 12486104
- PMCID: PMC2196071
- DOI: 10.1084/jem.20021405
Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling
Abstract
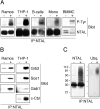
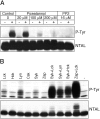
A key molecule necessary for activation of T lymphocytes through their antigen-specific T cell receptor (TCR) is the transmembrane adaptor protein LAT (linker for activation of T cells). Upon TCR engagement, LAT becomes rapidly tyrosine phosphorylated and then serves as a scaffold organizing a multicomponent complex that is indispensable for induction of further downstream steps of the signaling cascade. Here we describe the identification and preliminary characterization of a novel transmembrane adaptor protein that is structurally and evolutionarily related to LAT and is expressed in B lymphocytes, natural killer (NK) cells, monocytes, and mast cells but not in resting T lymphocytes. This novel transmembrane adaptor protein, termed NTAL (non-T cell activation linker) is the product of a previously identified WBSCR5 gene of so far unknown function. NTAL becomes rapidly tyrosine-phosphorylated upon cross-linking of the B cell receptor (BCR) or of high-affinity Fcgamma- and Fc epsilon -receptors of myeloid cells and then associates with the cytoplasmic signaling molecules Grb2, Sos1, Gab1, and c-Cbl. NTAL expressed in the LAT-deficient T cell line J.CaM2.5 becomes tyrosine phosphorylated and rescues activation of Erk1/2 and minimal transient elevation of cytoplasmic calcium level upon TCR/CD3 cross-linking. Thus, NTAL appears to be a structural and possibly also functional homologue of LAT in non-T cells.
Figures






References
-
- van Leeuwen, J.E., and L.E. Samelson. 1999. T cell antigen-receptor signal transduction. Curr. Opin. Immunol. 11:242–248. - PubMed
-
- Reth, M. 2001. Oligomeric antigen receptors: a new view on signaling for the selection of lymphocytes. Trends Immunol. 22:356–360. - PubMed
-
- Turner, H., and J.P. Kinet. 1999. Signalling through the high-affinity IgE receptor FcɛRI. Nature. 402:B24–B30. - PubMed
-
- Zhang, W., J. Sloan-Lancaster, J. Kitchen, R.P. Trible, and L.E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 92:83–92. - PubMed
-
- Zhang, W., R.P. Trible, M. Zhu, S.K. Liu, C.J. McGlade, and L.E. Samelson. 2000. Association of Grb2, Gads, and phospholipase C-γ1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J. Biol. Chem. 275:23355–23361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

