The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis
- PMID: 12486108
- PMCID: PMC2173988
- DOI: 10.1083/jcb.200205014
The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis
Abstract
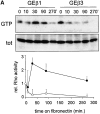
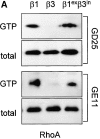
We have studied the formation of different types of cell matrix adhesions in cells that bind to fibronectin via either alpha5beta1 or alphavbeta3. In both cases, cell adhesion to fibronectin leads to a rapid decrease in RhoA activity. However, alpha5beta1 but not alphavbeta3 supports high levels of RhoA activity at later stages of cell spreading, which are associated with a translocation of focal contacts to peripheral cell protrusions, recruitment of tensin into fibrillar adhesions, and fibronectin fibrillogenesis. Expression of an activated mutant of RhoA stimulates alphavbeta3-mediated fibrillogenesis. Despite the fact that alpha5beta1-mediated adhesion to the central cell-binding domain of fibronectin supports activation of RhoA, other regions of fibronectin are required for the development of alpha5beta1-mediated but not alphavbeta3-mediated focal contacts. Using chimeras of beta1 and beta3 subunits, we find that the extracellular domain of beta1 controls RhoA activity. By expressing both beta1 and beta3 at high levels, we show that beta1-mediated control of the levels of beta3 is important for the distribution of focal contacts. Our findings demonstrate that the pattern of fibronectin receptors expressed on a cell dictates the ability of fibronectin to stimulate RhoA-mediated organization of cell matrix adhesions.
Figures















References
-
- Aota, S., M. Nomizu, and K. Yamada. 1994. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 269:24756–24761. - PubMed
-
- Arthur, W., L. Petch, and K. Burridge. 2000. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10:719–722. - PubMed
-
- Barry, S., H. Flinn, M. Humphries, D. Critchley, and A. Ridley. 1997. Requirement for Rho in integrin signalling. Cell Adhes. Commun. 4:387–398. - PubMed
-
- Berrier, A.L., R. Martinez, G.M. Bokoch, and S.L. LaFlamme. 2002. Integrin β tails activate Rac1. J. Cell Sci. 115:4285–4291 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

