Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli
- PMID: 12486111
- PMCID: PMC2173976
- DOI: 10.1083/jcb.200207071
Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli
Abstract
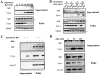
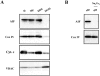
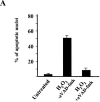
Mitochondrial outer membrane permeabilization by proapoptotic Bcl-2 family proteins, such as Bax, plays a crucial role in apoptosis induction. However, whether this only causes the intracytosolic release of inducers of caspase-dependent death, such as cytochrome c, or also of caspase-independent death, such as apoptosis-inducing factor (AIF) remains unknown. Here, we show that on isolated mitochondria, Bax causes the release of cytochrome c, but not of AIF, and the association of AIF with the mitochondrial inner membrane provides a simple explanation for its lack of release upon Bax-mediated outer membrane permeabilization. In cells overexpressing Bax or treated either with the Bax- or Bak-dependent proapoptotic drugs staurosporine or actinomycin D, or with hydrogen peroxide, caspase inhibitors did not affect the intracytosolic translocation of cytochrome c, but prevented that of AIF. These results provide a paradigm for mitochondria-dependent death pathways in which AIF cannot substitute for caspase executioners because its intracytosolic release occurs downstream of that of cytochrome c.
Figures











References
-
- Eskes, R., B. Antonsson, A. Osen-Sand, S. Montessuit, C. Richter, R. Sadoul, G. Mazzei, A. Nichols, and J.C. Martinou. 1998. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J. Cell Biol. 143:217–224. - PMC - PubMed
-
- Evan, G., and T. Littlewood. 1998. A matter of life and death. Science. 281:1317–1322. - PubMed
-
- Finucane, D.M., E. Bossy-Wetzel, N.J. Waterhouse, T.G.G. Cotter, and D.R. Green. 1999. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 274:2225–2233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

