Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1
- PMID: 12486114
- PMCID: PMC2173980
- DOI: 10.1083/jcb.200208050
Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1
Abstract
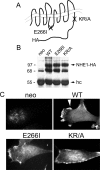
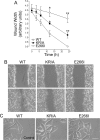
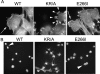
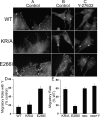
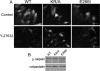
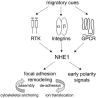
Directed cell movement is a multi-step process requiring an initial spatial polarization that is established by asymmetric stimulation of Rho GTPases, phosphoinositides (PIs), and actin polymerization. We report that the Na-H exchanger isoform 1 (NHE1), a ubiquitously expressed plasma membrane ion exchanger, is necessary for establishing polarity in migrating fibroblasts. In fibroblasts, NHE1 is predominantly localized in lamellipodia, where it functions as a plasma membrane anchor for actin filaments by its direct binding of ezrin/radixin/moesin (ERM) proteins. Migration in a wounding assay was impaired in fibroblasts expressing NHE1 with mutations that independently disrupt ERM binding and cytoskeletal anchoring or ion transport. Disrupting either function of NHE1 impaired polarity, as indicated by loss of directionality, mislocalization of the Golgi apparatus away from the orientation of the wound edge, and inhibition of PI signaling. Both functions of NHE1 were also required for remodeling of focal adhesions. Most notably, lack of ion transport inhibited de-adhesion, resulting in trailing edges that failed to retract. These findings indicate that by regulating asymmetric signals that establish polarity and by coordinating focal adhesion remodeling at the cell front and rear, cytoskeletal anchoring by NHE1 and its localized activity in lamellipodia act cooperatively to integrate cues for directed migration.
Figures
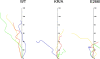

References
-
- Bernstein, B.W., W.B. Painter, H. Chen, L.S. Minamide, H. Abe, and J.R. Bamburg. 2000. Intracellular pH modulation of ADF/cofilin proteins. Cell Motil. Cytoskeleton. 47:319–336. - PubMed
-
- Bertrand, B., S. Wakabayashi, T. Ikeda, J. Pouyssegur, and M. Shigekawa. 1994. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J. Biol. Chem. 269:13703–13709. - PubMed
-
- Bialkowska, K., S. Kulkarni, X. Du, D.E. Goll, T.C. Saido, and J.E. Fox. 2000. Evidence that beta3 integrin-induced Rac activation involves the calpain-dependent formation of integrin clusters that are distinct from the focal complexes and focal adhesions that form as Rac and RhoA become active. J. Cell Biol. 151:685–696. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

