Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation
- PMID: 12486146
- PMCID: PMC6758409
- DOI: 10.1523/JNEUROSCI.22-24-10539.2002
Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation
Abstract
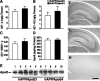
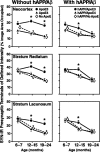
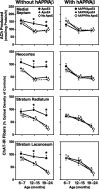
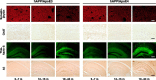
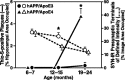
The most frequent human apolipoprotein (apo) E isoforms, E3 and E4, differentially affect Alzheimer's disease (AD) risk (E4 > E3) and age of onset (E4 < E3). Compared with apoE3, apoE4 promotes the cerebral deposition of amyloid beta (Abeta) peptides, which are derived from the amyloid precursor protein (APP) and play a central role in AD. However, it is uncertain whether Abeta deposition into plaques is the main mechanism by which apoE isoforms affect AD. We analyzed murine apoE-deficient transgenic mice expressing in their brains human APP (hAPP) and Abeta together with apoE3 or apoE4. Because cognitive decline in AD correlates better with decreases in synaptophysin-immunoreactive presynaptic terminals, choline acetyltransferase (ChAT) activity, and ChAT-positive fibers than with plaque load, we compared these parameters in hAPP/apoE3 and hAPP/apoE4 mice and singly transgenic controls at 6-7, 12-15, and 19-24 months of age. Brain aging in the context of high levels of nondeposited human Abeta resulted in progressive synaptic/cholinergic deficits. ApoE3 delayed the synaptic deficits until old age, whereas apoE4 was not protective at any of the ages analyzed. Old hAPP/apoE4 mice had more plaques than old hAPP/apoE3 mice, but synaptic/cholinergic deficits preceded plaque formation in hAPP/apoE4 mice. Moreover, despite their different plaque loads, old hAPP/apoE4 and hAPP/apoE3 mice had comparable synaptic/cholinergic deficits, and these deficits were found not only in the hippocampus but also in the neocortex, which in most mice contained no plaques. Thus, apoE3, but not apoE4, delays age- and Abeta-dependent synaptic deficits through a plaque-independent mechanism. This difference could contribute to the differential effects of apoE isoforms on the risk and onset of AD.
Figures
References
-
- Allen SJ, MacGowan SH, Tyler S, Wilcock GK, Robertson AGS, Holden PH, Smith SKF, Dawbarn D. Reduced cholinergic function in normal and Alzheimer's disease brain is associated with apolipoprotein E4 genotype. Neurosci Lett. 1997;239:33–36. - PubMed
-
- Berr C, Hauw J-J, Delaäre P, Duyckaerts C, Amouyel P. Apolipoprotein E allele 4 is linked to increased deposition of the amyloid β-peptide (A-β) in cases with or without Alzheimer's disease. Neurosci Lett. 1994;178:221–224. - PubMed
-
- Bierer L, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, Perl DP, Schmeidler J, Kanof P, Davis KL. Neurochemical correlates of dementia severity in Alzheimer's disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64:749–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
