Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina
- PMID: 12486148
- PMCID: PMC6758447
- DOI: 10.1523/JNEUROSCI.22-24-10558.2002
Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina
Abstract
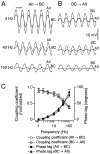
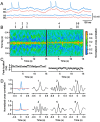
In the retina, AII (rod) amacrine cells are essential for integrating rod signals into the cone pathway. In addition to being interconnected via homologous gap junctions, these cells make extensive heterologous gap junctions with ON-cone bipolar cells (BCs). These gap junctions are the pathway for transfer of rod signals to the ON-system. To investigate the functional properties of these gap junctions, we performed simultaneous whole-cell recordings from pairs of AII amacrine cells and ON-cone bipolar cells in the in vitro slice preparation of the rat retina. We demonstrate strong electrical coupling with symmetrical junction conductance (approximately 1.2 nS) and very low steady-state voltage sensitivity. However, signal transmission is more effective in the direction from AII amacrine cells to ON-cone bipolar cells than in the other direction. This functional rectification can be explained by a corresponding difference in membrane input resistance between the two cell types. Signal transmission has low-pass filter characteristics with increasing attenuation and phase shift for increasing stimulus frequency. Action potentials in AII amacrine cells evoke distinct electrical postsynaptic potentials in ON-cone bipolar cells. Strong and temporally precise synchronization of subthreshold membrane potential fluctuations are commonly observed.
Figures




Similar articles
-
Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses.J Neurophysiol. 2005 Mar;93(3):1476-85. doi: 10.1152/jn.00597.2004. Epub 2004 Nov 3. J Neurophysiol. 2005. PMID: 15525810
-
AII (Rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina.Neuron. 2002 Mar 14;33(6):935-46. doi: 10.1016/s0896-6273(02)00609-8. Neuron. 2002. PMID: 11906699
-
Function and plasticity of homologous coupling between AII amacrine cells.Vision Res. 2004 Dec;44(28):3297-306. doi: 10.1016/j.visres.2004.07.012. Vision Res. 2004. PMID: 15535997
-
Electrical synapses between AII amacrine cells in the retina: Function and modulation.Brain Res. 2012 Dec 3;1487:160-72. doi: 10.1016/j.brainres.2012.05.060. Epub 2012 Jul 7. Brain Res. 2012. PMID: 22776293 Review.
-
Intrinsic properties and functional circuitry of the AII amacrine cell.Vis Neurosci. 2012 Jan;29(1):51-60. doi: 10.1017/S0952523811000368. Vis Neurosci. 2012. PMID: 22310372 Free PMC article. Review.
Cited by
-
Synaptic organization of the vertebrate retina: general principles and species-specific variations: the Friedenwald lecture.Invest Ophthalmol Vis Sci. 2010 Mar;51(3):1263-74. doi: 10.1167/iovs.09-4396. Invest Ophthalmol Vis Sci. 2010. PMID: 20185835 Free PMC article. No abstract available.
-
Dendrodendritic electrical synapses between mammalian retinal ganglion cells.J Neurosci. 2004 Nov 17;24(46):10553-67. doi: 10.1523/JNEUROSCI.3319-04.2004. J Neurosci. 2004. PMID: 15548670 Free PMC article.
-
Function and Plasticity of Electrical Synapses in the Mammalian Brain: Role of Non-Junctional Mechanisms.Biology (Basel). 2022 Jan 5;11(1):81. doi: 10.3390/biology11010081. Biology (Basel). 2022. PMID: 35053079 Free PMC article. Review.
-
A new player in circadian networks: Role of electrical synapses in regulating functions of the circadian clock.Front Physiol. 2022 Nov 3;13:968574. doi: 10.3389/fphys.2022.968574. eCollection 2022. Front Physiol. 2022. PMID: 36406999 Free PMC article. Review.
-
Active roles of electrically coupled bipolar cell network in the adult retina.J Neurosci. 2010 Jul 7;30(27):9260-70. doi: 10.1523/JNEUROSCI.1590-10.2010. J Neurosci. 2010. PMID: 20610761 Free PMC article.
References
-
- Bennett MVL. Electrical transmission: a functional analysis and comparison to chemical transmission. In: Kandel ER, editor. The handbook of physiology: the nervous system, Vol I. American Physiological Society; Washington, DC: 1977. pp. 357–416.
-
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Ret Eye Res. 2001;20:351–384. - PubMed
-
- Chun MH, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432. - PubMed
-
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol. 1995;361:461–478. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
