Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons
- PMID: 12486151
- PMCID: PMC6758411
- DOI: 10.1523/JNEUROSCI.22-24-10593.2002
Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons
Abstract
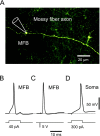
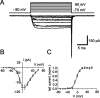
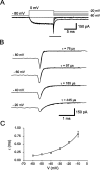
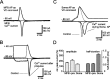
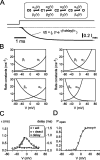
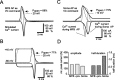
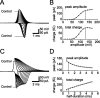
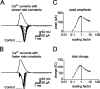
The presynaptic Ca2+ signal is a key determinant of transmitter release at chemical synapses. In cortical synaptic terminals, however, little is known about the kinetic properties of the presynaptic Ca2+ channels. To investigate the timing and magnitude of the presynaptic Ca2+ inflow, we performed whole-cell patch-clamp recordings from mossy fiber boutons (MFBs) in rat hippocampus. MFBs showed large high-voltage-activated Ca(2+) currents, with a maximal amplitude of approximately 100 pA at a membrane potential of 0 mV. Both activation and deactivation were fast, with time constants in the submillisecond range at a temperature of approximately 23 degrees C. An MFB action potential (AP) applied as a voltage-clamp command evoked a transient Ca2+ current with an average amplitude of approximately 170 pA and a half-duration of 580 microsec. A prepulse to +40 mV had only minimal effects on the AP-evoked Ca2+ current, indicating that presynaptic APs open the voltage-gated Ca2+ channels very effectively. On the basis of the experimental data, we developed a kinetic model with four closed states and one open state, linked by voltage-dependent rate constants. Simulations of the Ca2+ current could reproduce the experimental data, including the large amplitude and rapid time course of the current evoked by MFB APs. Furthermore, the simulations indicate that the shape of the presynaptic AP and the gating kinetics of the Ca2+ channels are tuned to produce a maximal Ca2+ influx during a minimal period of time. The precise timing and high efficacy of Ca2+ channel activation at this cortical glutamatergic synapse may be important for synchronous transmitter release and temporal information processing.
Figures

References
-
- Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–326. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
