Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice
- PMID: 12486156
- PMCID: PMC6758405
- DOI: 10.1523/JNEUROSCI.22-24-10633.2002
Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice
Abstract
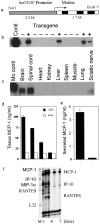
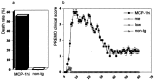
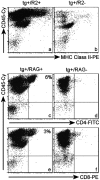
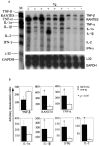
In this report we describe pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 (MCP-1) overexpression (PREMO), a novel animal model that exhibits features of human encephalopathic complications of inflammatory disorders such as viral meningoencephalitis and Lyme neuroborreliosis as well as the mild toxic encephalopathy that commonly precedes relapses of multiple sclerosis (MS). Overexpression of the mouse MCP-1 gene product (classically termed JE) in astrocytes, the major physiological CNS cellular source of MCP-1, failed to induce neurological impairment. Unexpectedly, transgenic (tg) mice overexpressing MCP-1 at a high level (MCP-1(hi)) manifested transient, severe encephalopathy with high mortality after injections of pertussis toxin (PTx) plus complete Freund's adjuvant (CFA). Surviving mice showed markedly improved function and did not relapse during a prolonged period of observation. Tg mice that expressed lower levels of MCP-1 were affected minimally after CFA/PTx injections, and tg expression of other chemokines failed to elicit this disorder. The disorder was significantly milder in mice lacking T-cells, which therefore play a deleterious role in this encephalopathic process. Disruption of CC chemokine receptor 2 (CCR2) abolished both CNS inflammation and encephalopathy, identifying CCR2 as a relevant receptor for this disorder. Proinflammatory and type 1 cytokines including TNF-alpha, IL-1beta, IFN-gamma, IL-2, RANTES, and IP-10 were elevated in CNS tissues from mice with PREMO. These studies characterize a novel model of reversible inflammatory encephalopathy that is dependent on both genetic and environmental factors.
Figures


Similar articles
-
Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS.J Neurosci. 2003 Aug 27;23(21):7922-30. doi: 10.1523/JNEUROSCI.23-21-07922.2003. J Neurosci. 2003. PMID: 12944523 Free PMC article.
-
Chronic expression of monocyte chemoattractant protein-1 in the central nervous system causes delayed encephalopathy and impaired microglial function in mice.FASEB J. 2005 May;19(7):761-72. doi: 10.1096/fj.04-3104com. FASEB J. 2005. PMID: 15857890
-
Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system.J Immunol. 2006 Nov 15;177(10):7242-9. doi: 10.4049/jimmunol.177.10.7242. J Immunol. 2006. PMID: 17082642
-
MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression.J Leukoc Biol. 1997 Jul;62(1):30-3. doi: 10.1002/jlb.62.1.30. J Leukoc Biol. 1997. PMID: 9225989 Review.
-
Targeting monocyte chemoattractant protein-1 signalling in disease.Expert Opin Ther Targets. 2003 Feb;7(1):35-48. doi: 10.1517/14728222.7.1.35. Expert Opin Ther Targets. 2003. PMID: 12556201 Review.
Cited by
-
Cerebrovascular Gi Proteins Protect Against Brain Hypoperfusion and Collateral Failure in Cerebral Ischemia.Mol Imaging Biol. 2023 Apr;25(2):363-374. doi: 10.1007/s11307-022-01764-8. Epub 2022 Sep 8. Mol Imaging Biol. 2023. PMID: 36074223 Free PMC article.
-
Abnormal immune response of CCR5-deficient mice to ocular infection with herpes simplex virus type 1.J Gen Virol. 2006 Mar;87(Pt 3):489-499. doi: 10.1099/vir.0.81339-0. J Gen Virol. 2006. PMID: 16476970 Free PMC article.
-
Inflammation in adult and neonatal stroke.Clin Neurosci Res. 2006 Dec 1;6(5):293-313. doi: 10.1016/j.cnr.2006.09.008. Clin Neurosci Res. 2006. PMID: 20300490 Free PMC article.
-
CCL2 transgene expression in the central nervous system directs diffuse infiltration of CD45(high)CD11b(+) monocytes and enhanced Theiler's murine encephalomyelitis virus-induced demyelinating disease.J Neurovirol. 2003 Dec;9(6):623-36. doi: 10.1080/13550280390247551. J Neurovirol. 2003. PMID: 14602575 Free PMC article.
-
Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS.J Neurosci. 2003 Aug 27;23(21):7922-30. doi: 10.1523/JNEUROSCI.23-21-07922.2003. J Neurosci. 2003. PMID: 12944523 Free PMC article.
References
-
- Aloisi F, Ria F, Columba-Cabezas S, Hess H, Penna G, Adorini L. Relative efficiency of microglia, astrocytes, dendritic cells, and B cells in naive CD4+ T-cell priming and Th1/Th2 cell restimulation. Eur J Immunol. 1999;29:2705–2714. - PubMed
-
- Berman JW, Guida MP, Warren J, Amat J, Brosnan CF. Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J Immunol. 1996;156:3017–3023. - PubMed
-
- Bohatschek M, Werner A, Raivich G. Systemic LPS injection leads to granulocyte influx into normal and injured brain: effects of ICAM-1 deficiency. Exp Neurol. 2001;172:137–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
