Modulation of the readily releasable pool of transmitter and of excitation-secretion coupling by activity and by serotonin at Aplysia sensorimotor synapses in culture
- PMID: 12486160
- PMCID: PMC6758440
- DOI: 10.1523/JNEUROSCI.22-24-10671.2002
Modulation of the readily releasable pool of transmitter and of excitation-secretion coupling by activity and by serotonin at Aplysia sensorimotor synapses in culture
Abstract
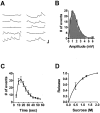
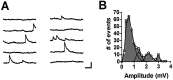
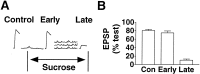
Short-term homosynaptic depression and heterosynaptic facilitation of transmitter release from mechanoreceptor sensory neurons of Aplysia are involved in habituation and sensitization, respectively, of defensive withdrawal reflexes. We investigated whether synaptic transmission is regulated in these forms of plasticity by means of changes in the size of the pool of transmitter available for immediate release [the readily releasable pool (RRP)] or in the efficacy of release from an unchanging pool. Using sensorimotor synapses formed in cell culture, we estimated the number of transmitter quanta in the RRP from the asynchronous release of neurotransmitter caused by application of a hypertonic bathing solution. Our experiments indicate that the transmitter released by action potentials and by hypertonic solution comes from the same pool. The RRP was reduced after homosynaptic depression of the EPSP by low-frequency stimulation and increased after facilitation of the EPSP by application of the endogenous facilitatory transmitter serotonin (5-HT) after homosynaptic depression. However, although the fractional changes in the RRP and in the EPSP were similar for both synaptic depression and facilitation when depression was induced by repeated hypertonic stimulation, the changes in the EPSP were significantly greater than the changes in the RRP when depression was induced by repeated electrical stimulation. These observations indicate that homosynaptic depression and restoration of depressed transmission by 5-HT are caused by changes in both the amount of transmitter available for immediate release and in processes involved in the coupling of the action potential to transmitter release.
Figures

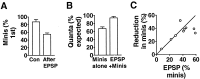


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
