Identification of an axotomy-induced glycosylated protein, AIGP1, possibly involved in cell death triggered by endoplasmic reticulum-Golgi stress
- PMID: 12486168
- PMCID: PMC6758406
- DOI: 10.1523/JNEUROSCI.22-24-10751.2002
Identification of an axotomy-induced glycosylated protein, AIGP1, possibly involved in cell death triggered by endoplasmic reticulum-Golgi stress
Abstract
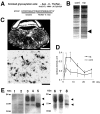
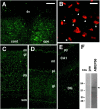
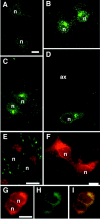
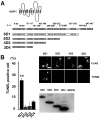
We developed a new method, designated N-linked glycosylation signal (NGS) differential display (DD)-PCR, that enables the identification of genes encoding N-linked glycosylated molecules that exhibit varying patterns of expression. Using this innovative technique, we identified an N-linked glycosylated 11-transmembrane domain protein that is upregulated in response to axotomy. Expression levels increased 3 d after axotomy, reached maximal levels at approximately postoperative days 5-7, and then gradually decreased through day 20. The protein was termed axotomy-induced glycosylated/Golgi-complex protein 1 (AIGP1). AIGP1 immunoreactivity is specifically localized in neurons, with subcellular localization within the Golgi, indicating that AIGP1 is a resident Golgi protein. Moreover, AIGP1 gene expression in cultured neurons is specifically induced by the endoplasmic reticulum (ER)-Golgi stressors tunicamycin and brefeldin A. We observed that the frequency of cell death is increased by AIGP1 overexpression and that the corresponding region of the protein implicated in the activity involves the large eighth and ninth transmembrane loops. Our results suggest that AIGP1 gene activation and protein accumulation in the Golgi complex in response to axotomy-induced ER-Golgi stress may contribute to signaling during programmed cell death in damaged neurons.
Figures



References
-
- Abeijon C, Hirschberg CB. Topography of glycosylation reactions in the endoplasmic reticulum. Trends Biochem Sci. 1992;17:32–36. - PubMed
-
- Aoki K, Sun YJ, Aoki S, Wada K, Wada E. Cloning, expression and mapping of a gene that is upregulated in adipose tissue of mice deficient in bombesin receptor subtype-3. Biochem Biophys Res Commun. 2002;290:1282–1288. - PubMed
-
- Baba N, Koji T, Itoh M, Mizuno A. Reciprocal changes in the expression of Bcl-2 and Bax in hypoglossal nucleus after axotomy in adult rats: possible involvement in the induction of neuronal cell death. Brain Res. 1999;827:122–129. - PubMed
-
- Cameron AA, Cliffer KD, Dougherty PM, Willis WD, Carlton SM. Changes in lectin, GAP-43 and neuropeptide staining in the rat superficial dorsal horn following experimental peripheral neuropathy. Neurosci Lett. 1991;131:249–252. - PubMed
-
- Cockcroft S. Mammalian phosphatidylinositol transfer proteins: emerging roles in signal transduction and vesicular traffic. Chem Phys Lipids. 1999;98:23–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
