Changes in mitochondrial status associated with altered Ca2+ homeostasis in aged cerebellar granule neurons in brain slices
- PMID: 12486169
- PMCID: PMC6758428
- DOI: 10.1523/JNEUROSCI.22-24-10761.2002
Changes in mitochondrial status associated with altered Ca2+ homeostasis in aged cerebellar granule neurons in brain slices
Abstract
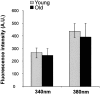
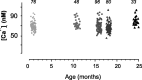
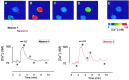
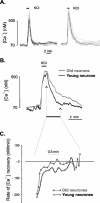
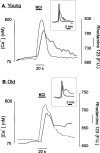
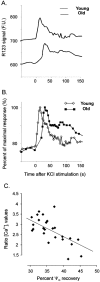
In the present work, we investigated the relationship between mitochondrial function and Ca2+ homeostasis in brain slices obtained from mice that aged normally. In acute preparations, the cerebellar neurons had similar values for intracellular free Ca2+ ([Ca2+]i) regardless of their age (range, 6 weeks to 24 months). However, compared with the young slices, the aged neurons (20-24 months) showed an enhanced rate of [Ca2+]i increases as a function of the time the slices were maintained in vitro. When slices were stimulated (KCl depolarization), there were significant differences in the patterns of [Ca2+]i signal displayed by the young and old cerebellar granule neurons. More importantly, the aged neurons showed a significant delay in their capacity to recover the resting [Ca2+]i. The relationship between [Ca2+]i and mitochondrial membrane potential was assessed by recording both parameters simultaneously, using fura-2 and rhodamine-123. In both young and aged neurons, the cytosolic [Ca2+]i signal was associated with a mitochondrial depolarization response. In the aged neurons, the mitochondria had a significantly longer repolarization response, and quantitative analysis showed a direct correlation between the delays in mitochondrial repolarization and [Ca2+]i recovery, indicating the causal relationship between the two parameters. Thus, the present results show that the reported changes in Ca2+ homeostasis associated with aging, which manifest principally in a decreased capacity of maintaining a stable resting [Ca2+]i or recovering the resting [Ca2+]i values after stimulation, are primarily attributable to a metabolic dysfunction in which the mitochondrial impairment plays an important role.
Figures



References
-
- Ames BN, Shigenaga MK, Hagen TM. Mitochondrial decay in aging. Biochim Biophys Acta. 1995;1271:165–170. - PubMed
-
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. - PubMed
-
- Campbell VA, Segurado R, Lynch MA. Regulation of intracellular Ca2+ concentration by interleukin-1β in rat cortical synaptosomes: an age-related study. Neurobiol Aging. 1998;19:575–579. - PubMed
-
- Cortopasi G, Wong A. Mitochondria in organismal aging and degeneration. Biochim Biophys Acta. 1999;1410:183–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
