Involvement of tissue plasminogen activator in onset and effector phases of experimental allergic encephalomyelitis
- PMID: 12486171
- PMCID: PMC4002885
- DOI: 10.1523/JNEUROSCI.22-24-10781.2002
Involvement of tissue plasminogen activator in onset and effector phases of experimental allergic encephalomyelitis
Abstract
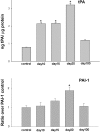
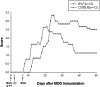
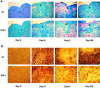
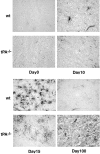
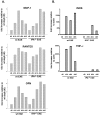
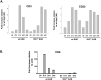
Inflammation, demyelination, and neurodegeneration are pathological features of multiple sclerosis (MS). In the brains of MS patients, tissue plasminogen activator (tPA) mRNA and protein are upregulated, and changes in the levels of tPA correlate with progression of the disease. However, the role of tPA in MS is as yet unknown. tPA functions in the CNS in neuronal plasticity and cell death. tPA also mediates the activation of microglia, the CNS "immune cells." In this study, we establish that tPA activity increases during major oligodendrocyte glycoprotein-induced experimental allergic encephalomyelitis (EAE) in normal mice. To explore the role of tPA in this disease as a model for MS, we have examined the EAE course and expression of histopathological markers in mice lacking tPA (tPA(-/-)). We find that tPA(-/-) mice have a delayed onset of EAE but then exhibit increased severity and delayed recovery from the neurological dysfunction. Demyelination and axon degeneration are delayed, microglial activation is attenuated, and the production of chemokines is decreased. Our results suggest that tPA and activated microglia have complex roles in MS/EAE, and that these roles are harmful during the onset of the disease but beneficial in the recovery phase. A temporally restricted attenuation of tPA activity could have therapeutic potential in the management of MS.
Figures

References
-
- Akenami F, Koskiniemi M, Mustjoki S, Siren V, Farkkila M, Vaheri A. Plasma and cerebrospinal fluid activities of tissue plasminogen activator and urokinase in multiple sclerosis. Fibrinolysis Proteolysis. 1997;11:109–113.
-
- Akenami F, Siren V, Wessman M, Koskiniemi M, Vaheri A. Tissue plasminogen activator gene expression in multiple sclerosis brain tissue. J Neurol Sci. 1999;165:71–76. - PubMed
-
- Andrade-Gordon P, Strickland S. Interaction of heparin with plasminogen activators and plasminogen: effects on the activation of plasminogen. Biochemistry. 1986;25:4033–4040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
