Isolation of relevant visual features from random stimuli for cortical complex cells
- PMID: 12486174
- PMCID: PMC6758424
- DOI: 10.1523/JNEUROSCI.22-24-10811.2002
Isolation of relevant visual features from random stimuli for cortical complex cells
Abstract
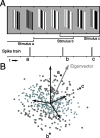
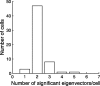
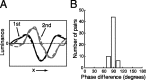
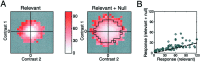
A crucial step in understanding the function of a neural circuit in visual processing is to know what stimulus features are represented in the spiking activity of the neurons. For neurons with complex, nonlinear response properties, characterization of feature representation requires measurement of their responses to a large ensemble of visual stimuli and an analysis technique that allows identification of relevant features in the stimuli. In the present study, we recorded the responses of complex cells in the primary visual cortex of the cat to spatiotemporal random-bar stimuli and applied spike-triggered correlation analysis of the stimulus ensemble. For each complex cell, we were able to isolate a small number of relevant features from a large number of null features in the random-bar stimuli. Using these features as visual stimuli, we found that each relevant feature excited the neuron effectively in isolation and contributed to the response additively when combined with other features. In contrast, the null features evoked little or no response in isolation and divisively suppressed the responses to relevant features. Thus, for each cortical complex cell, visual inputs can be decomposed into two distinct types of features (relevant and null), and additive and divisive interactions between these features may constitute the basic operations in visual cortical processing.
Figures



References
-
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A. 1985;2:284–299. - PubMed
-
- Albrecht DG, Geisler WS. Motion selectivity and the contrast-response function of simple cells in the visual cortex. Vis Neurosci. 1991;7:531–546. - PubMed
-
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparisons in visual neurons. Annu Rev Neurosci. 1985;8:407–430. - PubMed
-
- Anzai A, Ohzawa I, Freeman RD. Neural mechanisms for processing binocular information. I. Simple cells. J Neurophysiol. 1999;82:891–908. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
